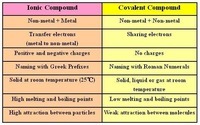
Covalent, Ionic, Transitional, and Polyatomic Naming
Quiz
•
Other Sciences
•
KG - University
•
Practice Problem
•
Hard

Charles Martinez
FREE Resource
Enhance your content in a minute
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
An ionic compound of calcium and chlorine would be named
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
A covalent compound made of one sulfur and two oxygen atoms would be named
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
An ionic compound made of copper (Cu2+) and oxygen would be named
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The covalent compound N2O5 would be named
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Because the overall charge in a compound must be ____________, the charge of iron in Fe2O3 can be calculated as 3+.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the compound TiO2, titanium has a charge of
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
The chemical formula for an ionic compound of aluminum and chlorine is
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Flocabulary: Mixtures & Compounds
Quiz
•
4th - 6th Grade

10 questions
Hort One 6.00 Review
Quiz
•
9th - 12th Grade

11 questions
Forensic Glass Analysis
Quiz
•
12th Grade - University

11 questions
Health & Wellness
Quiz
•
3rd - 6th Grade

10 questions
elements
Quiz
•
6th - 8th Grade

11 questions
Greenhouse Gases/ Ozone
Quiz
•
6th - 8th Grade

15 questions
Simple Machines
Quiz
•
6th Grade

13 questions
8th grade science
Quiz
•
8th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Other Sciences

4 questions
Conflict Resolution
Lesson
•
KG

20 questions
Place Value
Quiz
•
KG - 3rd Grade

20 questions
CVC Words
Quiz
•
KG - 1st Grade

24 questions
CKLA Unit 5 assessment K
Quiz
•
KG

10 questions
STAAR Review - Editing & Revising Clusters
Quiz
•
KG

10 questions
Reflexive Pronouns
Quiz
•
KG - 5th Grade

10 questions
Long i- igh, ie, and y Quiz
Quiz
•
KG - 3rd Grade

12 questions
Quarter Past, Half Past, and Quarter To
Quiz
•
KG - 12th Grade

