
Lewis Structure and Molecular Shapes
Authored by Lisa Thompson
Science
12th Grade
NGSS covered
Used 2+ times

AI Actions
Add similar questions
Adjust reading levels
Convert to real-world scenario
Translate activity
More...
Content View
Student View
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the correct Lewis Dot Structure for ammonia NH3?
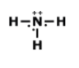
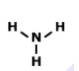
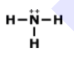
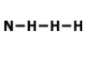
Tags
NGSS.HS-PS1-1
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
Pairs of valence electrons
Inner shell electrons
Mobile Electrons
Electrons closest to the nucleus
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Is this molecule symmetrical or asymmetrical?
Asymmetrical
Symmetrical
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Is this molecule symmetrical or asymmetrical?
Asymmetrical
Symmetrical
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

How many NON BONDING electron domains are around the central atom?
0
2
3
4
Tags
NGSS.HS-PS1-2
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What molecule below could be polar if it has polar bonds? Think about the symmetry of the molecule.




7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Which of the following is the correct Lewis structure for water (H2O)?
A) H-O-H with two lone pairs on oxygen
B) H=O=H with no lone pairs
C) H-O-H with one lone pair on oxygen
D) H-O-H with three lone pairs on oxygen
Tags
NGSS.HS-PS1-1
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

10 questions
DNA Quiz for 10th Grade Students
Quiz
•
10th Grade - University

10 questions
BAB 3 KELESTARIAN ALAM SEKITAR (IREADY)
Quiz
•
9th - 12th Grade

10 questions
Digestion
Quiz
•
12th Grade - University

10 questions
Properties of acid
Quiz
•
9th - 12th Grade

14 questions
1-5周期表タイムショック
Quiz
•
12th Grade

19 questions
T5 BAB 1 MIKROORGANISMA
Quiz
•
12th Grade

15 questions
Plant Tropisms
Quiz
•
9th - 12th Grade

18 questions
Ekološka globalizacija
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

29 questions
Alg. 1 Section 5.1 Coordinate Plane
Quiz
•
9th Grade

22 questions
fractions
Quiz
•
3rd Grade

11 questions
FOREST Effective communication
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Science

8 questions
Momentum and Collisions
Lesson
•
9th - 12th Grade

12 questions
The Respiratory System
Lesson
•
9th - 12th Grade

14 questions
Natural Selection and Adaptation
Lesson
•
9th - 12th Grade

10 questions
Symbiotic Relationships
Lesson
•
9th - 12th Grade

15 questions
Punnett Square & Pedigree Charts- Review
Quiz
•
9th - 12th Grade

28 questions
Asexual vs. Sexual Reproduction
Quiz
•
9th - 12th Grade

15 questions
Solar System
Lesson
•
9th - 12th Grade

40 questions
S2 Interim Review
Quiz
•
9th - 12th Grade