
Lewis Structure VSEPR
Quiz
•
Science
•
10th Grade
•
Practice Problem
•
Medium
Standards-aligned

Lisa Thompson
Used 2+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
According to VSEPR, molecules adjust their shapes to keep which of the following as far away as possible?
Pairs of valence electrons
Inner shell electrons
Mobile Electrons
Electrons closest to the nucleus
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
In the correct Lewis structure for CH4, how many unshared electron pairs surround the carbon?
2
8
0
4
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What is the correct Lewis structure for NH3?
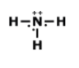
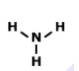
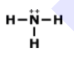
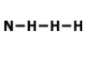
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

How many sigma bonds are in the nitrite ion, NO21-?
0
1
2
3
Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Which of the following is the correct Lewis structure for CH2O?
Option A
Option B
Option C
Option D
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

Draw the lewis dot structure for F2. Which of the following is the correct Lewis dot structure for the molecule F2?
A
B
C
D
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
How many electrons are shared in each bonding line?
1
2
3
4
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
The digestive system
Quiz
•
9th - 11th Grade

13 questions
Biology Semester Exam Review (1)
Quiz
•
9th - 12th Grade

10 questions
Quiz; Topographic (Contour) Mapping
Quiz
•
10th Grade

14 questions
Integrated Science
Quiz
•
9th - 11th Grade

10 questions
Agriculture
Quiz
•
10th - 12th Grade

16 questions
Tectonic plate movement
Quiz
•
9th - 12th Grade

18 questions
Sources of Energy
Quiz
•
7th - 10th Grade

15 questions
How Matter Can Change Quiz
Quiz
•
6th Grade - University
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade
Discover more resources for Science

10 questions
Exploring Plate Tectonics and Their Boundaries
Interactive video
•
6th - 10th Grade

70 questions
Advanced Biology Semester Review
Quiz
•
9th - 12th Grade

19 questions
ionic vs covalent
Quiz
•
9th - 12th Grade

27 questions
Macromolecules and Cellular Processes Quiz
Quiz
•
10th Grade

20 questions
UNIT 3 CFA
Quiz
•
10th Grade

64 questions
Honors December Holiday Break Assignment 2025
Quiz
•
10th Grade

10 questions
Comparing Light Waves and Sound Waves
Interactive video
•
6th - 10th Grade

10 questions
Claim Evidence Reasoning (CER) Essentials
Interactive video
•
6th - 10th Grade

