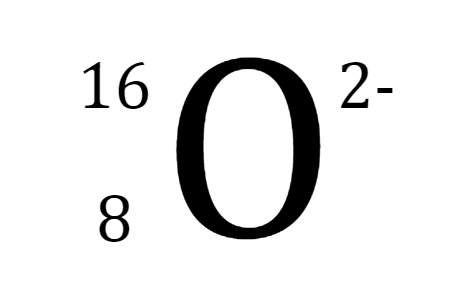Chemical Bonding
Quiz
•
Chemistry
•
9th Grade
•
Practice Problem
•
Medium
+1
Standards-aligned
Crissie Molskness
Used 6K+ times
FREE Resource
Enhance your content in a minute
15 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Typically, atoms are more stable when they are
bonded together
apart from each other
Tags
NGSS.HS-PS1-4
2.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Why do atoms bond?
They typically don't bond
To add or take away energy levels
To have a full valance shell.
To have a full inner shell
Tags
NGSS.HS-PS1-2
3.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Ionic bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
Depends on the situation
Tags
NGSS.HS-PS1-2
4.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
Covalent bonding is between a
nonmetal and nonmetal
metal and nonmetal
metal and metal
It depends on the situation
Tags
NGSS.HS-PS1-2
5.
MULTIPLE CHOICE QUESTION
1 min • 1 pt

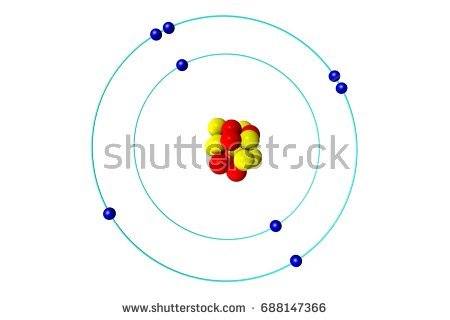
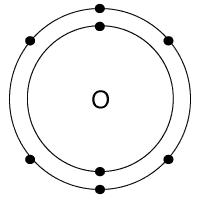
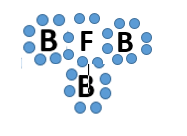
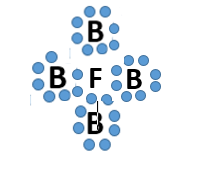
Which bond does this picture best represent?
Metallic bond
ionic bond
covalent bond
James Bond
6.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What do positive ions tend to do?
lose electrons
gain electrons
lose protons
gain protons
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
7.
MULTIPLE CHOICE QUESTION
1 min • 1 pt
What happens when magnesium loses 2 electrons?
It stabilizes to a net charge of 0
It turns into an atom
It becomes negatively charged
It becomes positively charged
Tags
NGSS.HS-PS1-1
NGSS.HS-PS1-2
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

12 questions
Funciones inorgánicas
Quiz
•
1st - 10th Grade

16 questions
C10.1 GCSE Reactions of Alkenes AQA 8462
Quiz
•
9th - 11th Grade

15 questions
Naming Ionic Compounds
Quiz
•
9th - 12th Grade

12 questions
Periodic Table and Trends
Quiz
•
9th - 11th Grade

17 questions
TEK 5C Periodic Trends
Quiz
•
9th - 12th Grade

15 questions
Year 9 chemistry revision (fun revise!)
Quiz
•
9th Grade

10 questions
Ligação Química + Eletricidade 9º
Quiz
•
9th Grade

18 questions
Simple Lewis Dot Structures
Quiz
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade

20 questions
Figurative Language Review
Quiz
•
6th Grade
Discover more resources for Chemistry

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Energy Transformations
Quiz
•
9th - 12th Grade

10 questions
Identifying Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

10 questions
Converting Grams to Moles: Mastering Molar Mass Calculations
Interactive video
•
6th - 10th Grade

21 questions
Introduction to Moles
Lesson
•
9th - 12th Grade

13 questions
Balanced Eqns & Cons of Mass
Quiz
•
9th - 12th Grade

11 questions
Practice with Mole Ratios
Lesson
•
9th - 12th Grade

23 questions
Ternary Ionic Compounds
Quiz
•
9th - 12th Grade