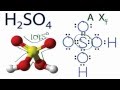
Molecular Geometry of H2SO4
Interactive Video
•
Lucas Foster
•
Chemistry
•
9th - 10th Grade
•
Hard
00:00
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE
30 sec • 1 pt
What theory is used to determine the molecular geometry of H2SO4?
2.
MULTIPLE CHOICE
30 sec • 1 pt
In the molecular structure of H2SO4, what color represents the oxygen atoms?
3.
MULTIPLE CHOICE
30 sec • 1 pt
What is the molecular geometry of H2SO4 as determined by the AXN notation?
4.
MULTIPLE CHOICE
30 sec • 1 pt
How many groups of atoms are attached to the central sulfur atom in H2SO4?
5.
MULTIPLE CHOICE
30 sec • 1 pt
What is the bond angle in a tetrahedral molecular geometry?
6.
MULTIPLE CHOICE
30 sec • 1 pt
Which atom is at the center of the H2SO4 molecule?
7.
MULTIPLE CHOICE
30 sec • 1 pt
What does the 'X' represent in the AXN notation for H2SO4?
8.
MULTIPLE CHOICE
30 sec • 1 pt
What is the significance of the bond angles in the molecular geometry of H2SO4?
9.
MULTIPLE CHOICE
30 sec • 1 pt
Which of the following is NOT a feature of the tetrahedral geometry?
10.
MULTIPLE CHOICE
30 sec • 1 pt
What is the role of the central sulfur atom in the molecular geometry of H2SO4?
Explore all questions with a free account
Popular Resources on Quizizz

5 questions
Brain Teasers
•
KG - University

37 questions
Math STAAR Review
•
4th Grade

12 questions
Earth Day
•
4th Grade

25 questions
STAAR REVIEW - SCIENCE
•
5th Grade

20 questions
Science STAAR Review! 23-24
•
5th Grade

20 questions
Reading Comprehension
•
5th Grade

20 questions
Types of Credit
•
9th - 12th Grade

23 questions
Math STAAR Review
•
4th Grade
Discover more resources for Chemistry

10 questions
Energy & Energy Transformations
•
9th - 12th Grade

20 questions
Chemical and Physical changes
•
10th - 12th Grade

62 questions
K Intro to REDOX pg1-4
•
10th Grade

15 questions
Endothermic and Exothermic Reactions
•
9th Grade

10 questions
Gas laws (Boyle's, Charles', Lussac)
•
9th Grade

52 questions
Unit 8: Gas Laws Review
•
10th Grade

23 questions
Boyle's, Charles', Gay-Lussac Laws
•
10th Grade

15 questions
Molarity & Dilutions
•
10th Grade