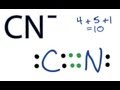
Valence Electrons and Ion Types
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of ion is the cyanide ion?
Cation
Anion
Radical
Neutral
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does nitrogen have?
3
6
4
5
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons available for the cyanide ion?
11
9
8
10
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in drawing the Lewis structure for the cyanide ion?
Checking for octets
Forming a bond between carbon and nitrogen
Placing electrons around nitrogen
Adding extra electrons to carbon
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does carbon have after the initial bond formation?
2
4
8
6
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What adjustment is made to satisfy the octet rule for carbon?
Moving electrons to form a triple bond
Adding a lone pair to carbon
Removing electrons from carbon
Adding more electrons to nitrogen
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How is the structural formula of the cyanide ion represented?
With a single line
With two lines
With four lines
With three lines
Similar Resources on Wayground

8 questions
Chromium and Cyanide Compounds
Interactive video
•
9th - 10th Grade

8 questions
Acid-Base Properties and Reactions
Interactive video
•
9th - 10th Grade

9 questions
Tin and Cyanide Compounds
Interactive video
•
9th - 10th Grade

11 questions
Understanding Lewis Structures and Covalent Bonding
Interactive video
•
9th - 10th Grade

6 questions
Beryllium and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in S2Cl2 Structure
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Electron Configuration
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in H3O+
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade