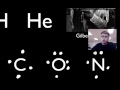
Drawing Lewis Dot Diagrams for Atoms and Molecules
Interactive Video
•
Science
•
6th - 10th Grade
•
Practice Problem
•
Easy
Standards-aligned
Jackson Turner
Used 3+ times
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic number of cesium?
53
54
56
55
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does cesium have?
1
4
2
3
Tags
NGSS.HS-PS1-1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group of elements on the periodic table is known for having eight valence electrons?
Transition metals
Halogens
Alkali metals
Noble gases
Tags
NGSS.HS-PS1-1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does neon have?
8
7
6
9
Tags
NGSS.HS-PS1-1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the correct Lewis dot structure for hydrogen?
H:
H
H.
H-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does oxygen have?
4
5
6
7
Tags
NGSS.HS-PS1-1
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a Lewis dot diagram, what does a single line between two atoms represent?
A single electron
A pair of electrons
A triple bond
An ionic bond
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Orangutan Communication and Rehabilitation
Interactive video
•
6th - 10th Grade

11 questions
Understanding the Periodic Table
Interactive video
•
6th - 10th Grade

11 questions
Calculating Volume of Prisms
Interactive video
•
6th - 10th Grade

11 questions
Exploring Electron Configuration and Orbital Notation
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

8 questions
2 Step Word Problems
Quiz
•
KG - University

20 questions
Comparing Fractions
Quiz
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Latin Bases claus(clois,clos, clud, clus) and ped
Quiz
•
6th - 8th Grade

22 questions
fractions
Quiz
•
3rd Grade

7 questions
The Story of Books
Quiz
•
6th - 8th Grade
Discover more resources for Science

12 questions
Newton's Laws of Motion
Lesson
•
6th - 8th Grade

20 questions
Elements, Compounds and Mixtures
Quiz
•
8th Grade

10 questions
Exploring Earth's Seasons and Their Causes
Interactive video
•
6th - 8th Grade

22 questions
Wave Properties
Lesson
•
7th Grade

8 questions
VP #9: Amoeba Sisters Pedigrees
Interactive video
•
7th Grade

20 questions
Cells! Cell Theory and Characteristics of Eukaryotes/Prokaryotes
Quiz
•
6th Grade

11 questions
The Water Cycle
Quiz
•
6th Grade

20 questions
Natural Selection and Adaptations
Quiz
•
6th - 8th Grade