Exploring Charge Group Dynamics
Interactive Video
•
Mathematics
•
6th - 10th Grade
•
Practice Problem
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge do alkali metals form when they become ions?
-1
+2
0
+1
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group of elements always forms a +2 charge when they become ions?
Alkaline earth metals
Halogens
Alkali metals
Noble gases
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of noble gases when they form ions?
They do not form ions
-1
+2
+1
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge do halogens form when they become ions?
-2
-1
+2
+1
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of ions formed by the oxygen family?
-1
-2
+2
+1
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If phosphorus forms an ion, what charge will it have?
-1
-2
-3
+3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
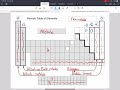
What is the significance of the demarcation line in the periodic table?
It separates alkali metals from alkaline earth metals
It separates elements with positive charges from those with negative charges
It separates noble gases from halogens
It separates metals from non-metals
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Finding Valence Electrons Made Easy
Interactive video
•
6th - 10th Grade

11 questions
Mastering Map Skills Techniques
Interactive video
•
6th - 10th Grade

11 questions
Balancing Chemical Equations Challenge
Interactive video
•
6th - 10th Grade

6 questions
Las reacciones redox
Interactive video
•
4th - 9th Grade

11 questions
Exploring the Science of Magnets
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

54 questions
Analyzing Line Graphs & Tables
Quiz
•
4th Grade

22 questions
fractions
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade

15 questions
Equivalent Fractions
Quiz
•
4th Grade
Discover more resources for Mathematics

22 questions
distributive property
Quiz
•
7th Grade

18 questions
Angle Relationships
Quiz
•
7th Grade

15 questions
Distributive Property & Review
Quiz
•
6th Grade

20 questions
Writing Algebraic Expressions
Quiz
•
6th Grade

20 questions
How Some Friendships Last — and Others Don’t Video Questions
Quiz
•
7th Grade

14 questions
finding slope from a graph
Quiz
•
8th Grade

20 questions
Ratios/Rates and Unit Rates
Quiz
•
6th Grade

18 questions
Handbook Refresher Quiz
Quiz
•
7th Grade