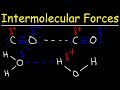
Exploring Intermolecular Forces and Their Impact on Boiling Point and Solubility
Interactive Video
•
Science
•
6th - 10th Grade
•
Hard
Standards-aligned
Lucas Foster
FREE Resource
Standards-aligned
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of interaction occurs between polar molecules like acetone?
Dipole-Dipole Interaction
Covalent Bonding
London Dispersion Forces
Ionic Bonding
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a dipole-dipole interaction, what attracts the carbon atom of one acetone molecule to the oxygen atom of another?
Similar charges
London dispersion forces
Opposite charges
Hydrogen bonds
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a special type of dipole-dipole interaction?
London Dispersion Forces
Ionic Bonding
Covalent Bonding
Hydrogen Bonding
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What effect does hydrogen bonding have on the boiling point of a molecule?
Depends on the molecule
No effect
Increases it
Decreases it
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which molecule has a higher boiling point due to hydrogen bonding?
Methane
Propane
Dimethyl Ether
Ethanol
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why does ethanol have a higher solubility in water compared to dimethyl ether?
Ethanol is nonpolar
Dimethyl ether is ionic
Dimethyl ether has hydrogen bonds
Ethanol has hydrogen bonds
Tags
NGSS.HS-PS2-6
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which molecule has a higher boiling point, ethanol or butanol?
Ethanol
Butanol
Neither has a boiling point
Both have the same boiling point
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Similar Resources on Wayground

6 questions
Stability and Chemical Bonds
Interactive video
•
6th - 9th Grade

6 questions
Stability and Chemical Bonds
Interactive video
•
6th - 9th Grade

11 questions
Exploring Pure Substances and Mixtures
Interactive video
•
6th - 10th Grade

11 questions
Exploring the Properties of Water: Polarity and Hydrogen Bonds
Interactive video
•
6th - 10th Grade

11 questions
Exploring Ionic and Covalent Bonds with Lewis Dot Diagrams
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

6 questions
FOREST Self-Discipline
Lesson
•
1st - 5th Grade

7 questions
Veteran's Day
Interactive video
•
3rd Grade

20 questions
Weekly Prefix check #2
Quiz
•
4th - 7th Grade
Discover more resources for Science

20 questions
Physical and Chemical Changes
Quiz
•
8th Grade

20 questions
Photosynthesis and Cellular Respiration
Quiz
•
7th Grade

18 questions
Interpreting Distance/Time Graphs
Quiz
•
6th Grade

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

20 questions
Energy Transformations Quiz
Quiz
•
6th Grade

20 questions
Conduction, Convection, & Radiation
Quiz
•
6th - 8th Grade

15 questions
Protein synthesis
Quiz
•
9th Grade

11 questions
8.6C Newton's Laws of Motion
Quiz
•
8th Grade