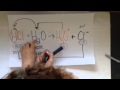
Exploring Conjugate Pairs in Mathematics
Interactive Video
•
Science
•
9th - 12th Grade
•
Hard
Jackson Turner
FREE Resource
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the definition of a Bronsted-Lowry acid?
A substance that donates a proton (H+).
A substance that accepts a proton (H+).
A substance that accepts an electron.
A substance that donates an electron.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What forms when hydrochloric acid is added to water?
Hydronium ion (H3O+) and Hydroxide ion (OH-).
Water molecules and Chloride ions.
Hydroxide ion (OH-) and Chloride ion (Cl-).
Hydronium ion (H3O+) and Chloride ion (Cl-).
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction between HCl and water, what role does water play?
Bronsted-Lowry acid
Bronsted-Lowry base
Catalyst
Solvent
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a conjugate base?
The original base before gaining a proton.
The original acid before losing a proton.
The species that forms when a base gains a proton.
The species that forms when an acid loses a proton.
5.
MULTIPLE SELECT QUESTION
30 sec • 1 pt
Which pair is an example of a conjugate acid-base pair?
HCl and H2O
HCl and Cl-
Cl- and H3O+
H2O and H3O+
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does it mean if two species differ by one proton?
They are isotopes.
They are resonance structures.
They are conjugate acid-base pairs.
They are structural isomers.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the Bronsted-Lowry acid in a reaction?
It gains a proton.
It loses a proton.
It remains unchanged.
It becomes a catalyst.
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Chemical Reactions and Equations
Interactive video
•
9th - 12th Grade

11 questions
Understanding Acids and Bases
Interactive video
•
9th - 12th Grade

11 questions
Exploring Acid-Base Theories: Brønsted-Lowry and Lewis
Interactive video
•
9th - 12th Grade

11 questions
pH and pOH Calculations in Chemistry
Interactive video
•
10th - 12th Grade

11 questions
Understanding Amphiprotic Substances
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Chemistry and pH Calculations
Interactive video
•
10th - 12th Grade

11 questions
Understanding the Henderson-Hasselbalch Equation
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Theories and Concepts
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Science

16 questions
Metric Conversions
Quiz
•
11th Grade

10 questions
Exploring the Scientific Method
Interactive video
•
6th - 10th Grade

10 questions
Exploring Chemical and Physical Changes
Interactive video
•
6th - 10th Grade

17 questions
Enzymes
Quiz
•
9th Grade

10 questions
Exploring Newton's Laws of Motion
Interactive video
•
6th - 10th Grade

21 questions
Cell Organelles
Quiz
•
9th Grade

10 questions
Exploring Metals, Nonmetals, and Metalloids
Interactive video
•
6th - 10th Grade

10 questions
Tonicity BR
Quiz
•
9th Grade