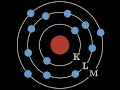
Electron Shells and Their Properties
Interactive Video
•
Physics, Chemistry, Science
•
7th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the Electron Shell Model help us understand about atoms?
The atomic mass of an element
The number of protons in an atom
The chemical properties of an element
The distribution of electrons in an atom
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the electrons in the outermost shell of an atom called?
Bonding electrons
Valence electrons
Free electrons
Core electrons
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which shell is known as the K-shell?
The third shell
The outermost shell
The innermost shell
The second shell
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons can the L-shell hold at maximum?
8 electrons
2 electrons
18 electrons
32 electrons
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula to calculate the maximum number of electrons a shell can hold?
n_max = 2n
n_max = n^2
n_max = n + 2
n_max = 2n^2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
For the third shell, what is the value of 'n' in the formula for maximum electrons?
1
4
2
3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many electrons does a hydrogen atom have?
3 electrons
4 electrons
2 electrons
1 electron
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Exploring the Octet Rule and Valence Charges
Interactive video
•
6th - 10th Grade

11 questions
Periodic Table Trends Exploration
Interactive video
•
6th - 10th Grade

11 questions
Exploring the Bohr Model of the Atom
Interactive video
•
6th - 10th Grade

11 questions
Exploring Bohr Models in Chemistry
Interactive video
•
6th - 10th Grade

11 questions
Exploring Periodic Table Trends and Patterns
Interactive video
•
6th - 10th Grade

11 questions
Exploring Ions and Bonding Types
Interactive video
•
6th - 10th Grade

11 questions
Exploring Ionic and Covalent Bonds
Interactive video
•
6th - 10th Grade

11 questions
Periodic Trends in Chemistry: Day 2 Insights
Interactive video
•
6th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

20 questions
Calculating Net Force
Quiz
•
6th - 9th Grade

10 questions
Density Test
Quiz
•
8th Grade

15 questions
Position vs. Time and Velocity vs. Time Graphs
Quiz
•
10th - 12th Grade

10 questions
Using Scalar and Vector Quantities
Quiz
•
8th - 12th Grade

19 questions
Distance time graphs
Quiz
•
8th Grade

20 questions
Acceleration
Quiz
•
9th Grade

5 questions
Reading Motion Graphs
Lesson
•
8th - 10th Grade