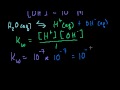
Understanding Water Chemistry and Autoionization
Interactive Video
•
Chemistry, Science
•
10th - 12th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is responsible for holding water molecules together in liquid and solid states?
Hydrogen bond
Metallic bond
Covalent bond
Ionic bond
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the process called when water molecules spontaneously ionize to form hydronium and hydroxide ions?
Hydrolysis
Autoionization
Neutralization
Condensation
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the autoionization of water, what is the equilibrium constant (Kw) at room temperature?
10^-7
10^-10
10^-3
10^-14
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'p' in pH and pKw stand for in chemistry?
Potential
Power
Partial
Pressure
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the pH of pure water at room temperature?
3
7
14
10
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does an increase in hydrogen ion concentration affect the pH of a solution?
Decreases pH
Neutralizes pH
Increases pH
No effect on pH
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the pOH of pure water at room temperature?
14
10
7
3
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Autoionization of Water and pH Concepts
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
10th - 12th Grade

11 questions
Exploring Acids and Bases in Chemistry
Interactive video
•
9th - 12th Grade

11 questions
Understanding Sulfuric Acid and pH Relationships
Interactive video
•
10th - 12th Grade

11 questions
pH Stability and Buffer Systems in Biological Proteins
Interactive video
•
10th - 12th Grade

11 questions
Le Chatelier's Principle and Solubility
Interactive video
•
10th - 12th Grade

11 questions
Titration of Weak Acids
Interactive video
•
10th - 12th Grade

11 questions
Titration Curve Analysis
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade