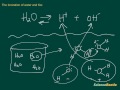
Ionization of Water Concepts
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of the video tutorial?
The chemical reactions of hydrogen
The ionization of water and the water constant KW
The structure of the water molecule
The properties of acids and bases
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the ionization of water produce?
Hydrogen ions and hydroxide ions
Carbon dioxide and water
Oxygen and hydrogen gas
Salt and water
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the water molecule considered polar?
Because it is a gas at room temperature
Because it has a positive and a negative end
Because it forms a solid at low temperatures
Because it is a non-conductor of electricity
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when another water molecule interacts with a water molecule in solution?
It releases oxygen gas
It becomes a non-polar molecule
It forms a solid precipitate
It forms hydrogen and hydroxide ions
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of the equilibrium process in the ionization of water?
The solution becomes basic
The solution becomes acidic
The water completely ionizes
The rate of ion formation equals the rate of water formation
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the ionization constant KW represent?
The ratio of the concentrations of hydrogen and hydroxide ions
The difference between the concentrations of hydrogen and hydroxide ions
The sum of the concentrations of hydrogen and hydroxide ions
The product of the concentrations of hydrogen and hydroxide ions
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the value of the ionization constant KW at 25°C?
1 * 10^-14
1 * 10^-7
1 * 10^-12
1 * 10^-10
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
KW and Temperature Relationships
Interactive video
•
10th - 12th Grade

11 questions
Water Ionization and pH Relationship in Chemistry
Interactive video
•
9th - 12th Grade

11 questions
Water Dissociation and pH Concepts
Interactive video
•
10th - 12th Grade

11 questions
Understanding pH and Acidity Concepts
Interactive video
•
10th - 12th Grade

11 questions
Autoionization of Water and pH Concepts
Interactive video
•
10th - 12th Grade

11 questions
Understanding Water Chemistry and Autoionization
Interactive video
•
10th - 12th Grade

11 questions
Hydronium and Hydroxide Ion Concepts
Interactive video
•
10th - 12th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab safety
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

12 questions
Counting Significant Figures Quick Check
Quiz
•
9th - 12th Grade

10 questions
Significant Figures Int 2
Quiz
•
9th - 12th Grade

19 questions
States of Matter Review
Quiz
•
10th Grade

21 questions
Lab Safety
Quiz
•
10th Grade