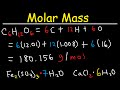
Molar Mass and Formula Weight Concepts
Interactive Video
•
Chemistry, Science
•
9th - 12th Grade
•
Easy
Ethan Morris
Used 2+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of carbon as mentioned in the video?
44.01
12.01
6
16
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the molar mass of CO2?
Subtract the atomic mass of oxygen from carbon
Multiply the atomic mass of carbon by two
Add the atomic masses of one carbon and two oxygen atoms
Divide the atomic mass of carbon by the atomic mass of oxygen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of methane (CH4) as calculated in the video?
44.01 g/mol
58.44 g/mol
18.016 g/mol
16.042 g/mol
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula weight of sodium chloride (NaCl)?
39.998 g/mol
58.44 g/mol
18.016 g/mol
342.296 g/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is true about formula weight?
It is only used for molecular compounds
It is not related to the periodic table
It is calculated by subtracting atomic masses
It is the same as molar mass for ionic compounds
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of glucose (C6H12O6) as calculated in the video?
342.296 g/mol
525.992 g/mol
219.076 g/mol
180.156 g/mol
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbon atoms are in sucrose (C12H22O11)?
6
11
12
22
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Exploring Moles and Molar Mass Concepts
Interactive video
•
9th - 12th Grade

11 questions
Understanding Moles and Atomic Mass
Interactive video
•
9th - 12th Grade

11 questions
Empirical Formulas and Mass Spectrometry
Interactive video
•
10th - 12th Grade

11 questions
Understanding Moles in Chemistry
Interactive video
•
9th - 12th Grade

11 questions
Understanding Moles and Molar Mass
Interactive video
•
8th - 12th Grade

11 questions
Mass Percentages and Molar Masses in Chemistry
Interactive video
•
9th - 12th Grade

9 questions
Molar Mass and Chemical Formulas
Interactive video
•
10th - 12th Grade

11 questions
Calculating Atoms in Molecules and Compounds
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade