Cathode Ray Tube Experiment Quiz
Interactive Video
•
Physics, Science
•
9th - 12th Grade
•
Medium
Lucas Foster
Used 2+ times
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What was the primary discovery made by J.J. Thompson using the cathode ray tube experiment?
The existence of protons
The presence of electrons in atoms
The structure of the nucleus
The concept of atomic mass
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
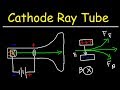
Why is it important to maintain a low pressure inside the cathode ray tube?
To increase the speed of the electrons
To prevent the tube from overheating
To ensure the electron beam is visible
To allow the electrons to collide with gas particles
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the role of the anode in the cathode ray tube?
To create a magnetic field
To heat the cathode
To emit electrons
To attract electrons
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the experiment called the 'cathode ray tube' experiment?
Because the anode emits the rays
Because the cathode is positively charged
Because the electron beam originates from the cathode
Because the tube is made of cathode material
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How did J.J. Thompson determine that the cathode ray consists of negatively charged particles?
By measuring the temperature of the cathode
By changing the pressure inside the tube
By using a magnet to deflect the beam
By observing the color of the glow
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What conclusion did Thompson draw from using different cathode materials?
The cathode material affects the speed of electrons
Cathode rays are unique to each metal
All atoms contain negatively charged particles
Only certain metals can emit cathode rays
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the radius of curvature in the cathode ray tube experiment?
It determines the color of the glow
It affects the pressure inside the tube
It helps calculate the charge-to-mass ratio
It changes the speed of the electrons
Create a free account and access millions of resources
Similar Resources on Wayground

3 questions
TED-Ed: How X-rays see through your skin - Ge Wang
Interactive video
•
KG - University

11 questions
Understanding New Technologies
Interactive video
•
9th - 12th Grade

11 questions
X-ray Tube Components and Functions
Interactive video
•
9th - 12th Grade

11 questions
Coolidge Tube Function and Properties
Interactive video
•
9th - 12th Grade

6 questions
Light in a Tube: Exploring Discharge Tube Experiments
Interactive video
•
10th Grade - University

6 questions
TED-Ed: How X-rays see through your skin - Ge Wang
Interactive video
•
KG - University

11 questions
History and Evolution of Video Technology
Interactive video
•
9th - 12th Grade

3 questions
J.J. Thomson's Charge-to-Mass Ratio Experiment
Interactive video
•
10th Grade - University
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Physics

20 questions
Position vs. Time Graphs
Quiz
•
9th Grade

25 questions
Newton's Laws of Motion
Quiz
•
9th Grade

20 questions
Claim Evidence Reasoning
Quiz
•
9th - 12th Grade

14 questions
Distance & Displacement
Quiz
•
11th Grade

17 questions
Free Body Diagrams
Quiz
•
9th - 12th Grade

10 questions
Exit Check 3.3 - Universal Gravitation
Quiz
•
9th Grade

20 questions
Motion Graphs
Quiz
•
11th - 12th Grade

10 questions
Exit Check 3.4 - Moon's Orbit
Quiz
•
9th Grade