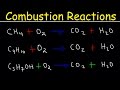
Combustion Reactions and Balancing Hydrocarbon Equations
Interactive Video
•
Chemistry, Science, Physics
•
9th - 12th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the typical products of a complete combustion reaction involving hydrocarbons?
Carbon dioxide and water
Carbon monoxide and water
Hydrogen and carbon
Methane and oxygen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In a complete combustion reaction, what is the first step in balancing the equation?
Balance the hydrogen atoms
Balance the oxygen atoms
Balance the nitrogen atoms
Balance the carbon atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When balancing the combustion of propane, how many carbon dioxide molecules are produced?
One
Two
Three
Four
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of oxygen atoms on the right side when balancing the combustion of propane?
Eight
Ten
Twelve
Fourteen
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you handle fractions when balancing the combustion of ethane?
Add more oxygen molecules
Ignore them
Multiply all coefficients by two
Leave them as they are
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in balancing the combustion of butane?
Balance the hydrogen atoms
Balance the nitrogen atoms
Balance the carbon atoms
Balance the oxygen atoms
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When balancing the combustion of propanol, what is the total number of hydrogen atoms on both sides?
Eight
Twenty
Twelve
Sixteen
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Popular Resources on Wayground

8 questions
2 Step Word Problems
Quiz
•
KG - University

20 questions
Comparing Fractions
Quiz
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Latin Bases claus(clois,clos, clud, clus) and ped
Quiz
•
6th - 8th Grade

22 questions
fractions
Quiz
•
3rd Grade

7 questions
The Story of Books
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Unit 7 Chemical Reactions
Quiz
•
10th Grade

20 questions
Elements, Compounds, and Mixtures
Quiz
•
10th Grade

20 questions
Practice: E-Con, Orbital Notation, Noble Gas Notation
Quiz
•
10th Grade

20 questions
Stoichiometry Practice
Quiz
•
10th Grade

22 questions
Periodic Trends Freshman
Quiz
•
10th Grade

23 questions
Unit 7 Chemical Reactions
Quiz
•
10th Grade

17 questions
Reaction Rates
Quiz
•
11th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade