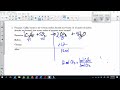
Balancing Combustion Reactions of Propane and Stoichiometry Calculations
Interactive Video
•
Chemistry, Mathematics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main product formed when propane undergoes combustion in air?
Carbon Dioxide
Nitrogen
Methane
Sulfur Dioxide
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element should you start with when balancing the combustion equation of propane?
Oxygen
Hydrogen
Carbon
Nitrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If 12 moles of carbon dioxide are produced, how many moles of propane were burned?
4 moles
5 moles
3 moles
2 moles
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the stoichiometric ratio of propane to carbon dioxide in the combustion reaction?
1:2
1:3
1:4
1:5
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of oxygen are required to completely burn 4 moles of propane?
10 moles
15 moles
20 moles
25 moles
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the stoichiometric ratio of propane to oxygen in the combustion reaction?
1:5
1:4
1:3
1:2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of water are produced when 12 moles of carbon dioxide are formed?
18 moles
14 moles
12 moles
16 moles
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Gas Laws and Chemical Reactions
Interactive video
•
10th - 12th Grade

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 12th Grade

11 questions
Thermodynamics Principles and Energy Transfer in Chemical Systems
Interactive video
•
9th - 12th Grade

11 questions
Mastering Moles and Stoichiometry in Chemistry
Interactive video
•
9th - 10th Grade

11 questions
Thermochemical Equations and Energy Conversions Quiz
Interactive video
•
10th - 12th Grade

10 questions
Balancing Combustion Reactions of Propane
Interactive video
•
9th - 10th Grade

11 questions
Chemical Calculations and Concepts
Interactive video
•
9th - 12th Grade

11 questions
Limiting Reactants and Percent Yield
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade