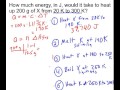
Heat Transfer and Properties of Substance X
Interactive Video
•
Chemistry, Physics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the melting point of substance X?
190 Kelvin
250 Kelvin
300 Kelvin
150 Kelvin
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is the specific heat of substance X in its liquid form?
2.00 J/gK
1.11 J/gK
0.67 J/gK
3.67 J/gK
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in heating substance X from 20K to 300K?
Heat from 250K to 300K
Heat from 20K to 190K
Melt at 190K
Boil at 250K
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much heat is required to raise the temperature of solid X from 20K to 190K?
36,700 J
80,000 J
12,000 J
37,740 J
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of substance X?
10 g/mol
16 g/mol
12.5 g/mol
20 g/mol
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much heat is required to melt 16 moles of substance X?
36,700 J
80,000 J
37,740 J
12,000 J
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the specific heat of substance X in its gaseous form?
0.67 J/gK
1.11 J/gK
3.67 J/gK
2.00 J/gK
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Heating Curve Calculations and Concepts
Interactive video
•
9th - 12th Grade

11 questions
Heating Curve Calculations for Water
Interactive video
•
9th - 12th Grade

11 questions
Heating Curve and Phase Transitions
Interactive video
•
9th - 12th Grade

11 questions
Heat Transfer and Phase Changes
Interactive video
•
9th - 12th Grade

11 questions
Latent Heat and State Changes
Interactive video
•
9th - 12th Grade

11 questions
Thermodynamics of Phase Changes
Interactive video
•
9th - 12th Grade

11 questions
Understanding Heating and Cooling Curves of Water
Interactive video
•
9th - 12th Grade

11 questions
Heat Transfer and Specific Heat
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade