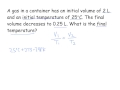
Gas Laws and Temperature Conversions
Interactive Video
•
Mathematics, Physics, Science
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial volume of the gas in the problem statement?
1 liter
3 liters
0.5 liters
2 liters
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the initial temperature of the gas in Celsius?
0 degrees
25 degrees
50 degrees
100 degrees
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula used in Charles Law?
P1/T1 = P2/T2
V1/T1 = V2/T2
V1*P1 = V2*P2
P1*V1 = P2*V2
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert Celsius to Kelvin?
Add 273
Add 100
Multiply by 2
Subtract 273
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final volume of the gas in the problem?
2 liters
0.25 liters
0.5 liters
1 liter
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in solving for the final temperature?
Cross-multiply
Divide pressures
Add volumes
Subtract temperatures
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final temperature in Kelvin after calculation?
100 K
298 K
74.5 K
37.25 K
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What operation is performed to convert the final temperature from Kelvin to Celsius?
Divide by 2
Add 273
Subtract 273
Multiply by 2
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final temperature in Celsius?
-37.25 degrees
0 degrees
-235.75 degrees
25 degrees
Similar Resources on Wayground

6 questions
Understanding Absolute Zero and Gases
Interactive video
•
9th - 10th Grade

11 questions
Thermal Equilibrium and Specific Heat
Interactive video
•
9th - 10th Grade

11 questions
Gas Law Concepts and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Temperature Conversion and Scales
Interactive video
•
9th - 10th Grade

11 questions
Mastering Temperature Scales and Conversions in Science
Interactive video
•
9th - 10th Grade

11 questions
Charles's Law and Gas Behavior
Interactive video
•
9th - 10th Grade
![General Chemistry | Ideal Gas Law (PV=nRT) [Example #1]](https://cf.quizizz.com/image/image-loader.svg)
8 questions
General Chemistry | Ideal Gas Law (PV=nRT) [Example #1]
Interactive video
•
9th - 10th Grade

11 questions
Ideal Gas Law Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Mathematics

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade

15 questions
Adding and Subtracting Polynomials
Quiz
•
9th Grade

20 questions
1.1 (a) Classifying Polynomials
Quiz
•
9th Grade

12 questions
Classifying Polys - 1.1
Quiz
•
10th - 12th Grade

20 questions
Function or Not? Domain and Range
Quiz
•
9th - 12th Grade

20 questions
Order of Operations
Quiz
•
9th Grade

19 questions
Constructions Review SKG
Quiz
•
10th Grade