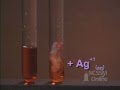
Chemical Equilibrium and Reaction Effects
Interactive Video
•
Chemistry, Science, Biology
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does L's principle suggest about a reaction at equilibrium when stress is applied?
The reaction will slow down.
The reaction will speed up.
The reaction will shift to relieve the stress.
The reaction will stop.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to the color of the solution when iron(III) nitrate is added?
It turns blue.
It becomes colorless.
It turns a darker red.
It turns green.
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the addition of potassium thiocyanate affect the reaction?
The reaction becomes colorless.
The reaction stops.
The reaction shifts in the forward direction.
The reaction shifts in the reverse direction.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the effect of adding sodium hydroxide to the reaction mixture?
The red color intensifies.
The red color disappears completely.
The solution turns blue.
The red color decreases significantly.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when potassium chloride is added to the solution?
The color intensity increases.
The color intensity decreases.
The solution turns green.
The solution becomes colorless.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is observed when silver nitrate is added to the reaction mixture?
The solution turns blue.
The red color disappears and a white solid forms.
The red color intensifies.
The solution becomes colorless.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the formation of a white solid indicate when silver nitrate is added?
Formation of a new complex ion.
Precipitation of iron ions.
Formation of slightly soluble silver thiocyanate.
Complete dissolution of reactants.
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Act: Exploring Le Chatelier's Principle in Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Equilibrium Systems and Reaction Dynamics
Interactive video
•
9th - 10th Grade

11 questions
Comparing Reaction Quotients and Equilibrium
Interactive video
•
9th - 10th Grade

11 questions
Equilibrium Shifts and Le Chatelier's Principle
Interactive video
•
9th - 10th Grade

11 questions
Le Chatelier's Principle and Equilibrium
Interactive video
•
9th - 10th Grade

6 questions
Chemical Equilibrium and Color Changes
Interactive video
•
9th - 10th Grade

11 questions
Equilibrium Color Changes and Reactions
Interactive video
•
9th - 10th Grade

8 questions
Thiocyanate and Mercury Chemistry
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade