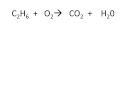
Balancing Hydrocarbon Combustion Reactions
Interactive Video
•
Chemistry, Science, Physics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary goal when balancing hydrocarbon combustion reactions?
To reduce the number of reactants
To increase the number of products
To make sure the number of atoms for each element is equal on both sides
To ensure the equation is aesthetically pleasing
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the combustion of methane, what is the first element you should balance?
Hydrogen
Nitrogen
Carbon
Oxygen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogens are on the left side of the methane combustion equation?
Six
Four
Two
Eight
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When balancing propane combustion, how many carbon atoms are on the left side?
One
Three
Four
Two
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the coefficient for water when balancing hydrogen in propane combustion?
Five
Two
Three
Four
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many total oxygen atoms are needed on the right side of the propane combustion equation?
Eight
Ten
Fourteen
Twelve
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is a common issue when balancing reactions with an odd number of hydrogens?
It results in an odd number of oxygens
It makes the reaction impossible to balance
It requires more reactants
It leads to an excess of products
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Balancing Combustion Reactions
Interactive video
•
9th - 10th Grade

11 questions
Balancing Combustion Reactions Concepts
Interactive video
•
10th Grade

11 questions
Balancing Combustion Reactions
Interactive video
•
9th - 10th Grade

8 questions
Combustion Reactions and Hydrocarbons
Interactive video
•
9th - 10th Grade

11 questions
Mastering Combustion Reactions in Chemistry
Interactive video
•
9th - 10th Grade

11 questions
Properties and Combustion of Alkanes
Interactive video
•
9th - 10th Grade

11 questions
Balancing Chemical Equations
Interactive video
•
9th - 10th Grade

11 questions
Balancing Combustion Reactions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

20 questions
ELA Advisory Review
Quiz
•
7th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Naming Covalent and Ionic Compounds
Quiz
•
10th Grade

43 questions
Electron Configuration and Orbital Notation
Quiz
•
10th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

20 questions
Electron Configuration & Orbital Notation
Quiz
•
9th - 12th Grade