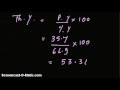
Mole Ratios and Yield Calculations
Interactive Video
•
Chemistry, Mathematics, Science
•
9th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the relationship between theoretical yield, practical yield, and percent yield?
Theoretical yield is the product of practical yield and percent yield.
Theoretical yield is the sum of practical yield and percent yield.
Theoretical yield is the quotient of practical yield and percent yield.
Theoretical yield is the difference between practical yield and percent yield.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the practical yield is 35.7 and the percent yield is 66.9, what is the theoretical yield?
2.38
3.34
4.56
5.67
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you convert grams to moles?
Multiply the mass by the molar mass.
Divide the mass by the molar mass.
Add the mass to the molar mass.
Subtract the molar mass from the mass.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the mole ratio of AlBr3 to Br2 in the given reaction?
1:1
2:3
1:2
3:2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of establishing a mole ratio in a chemical reaction?
To find the amount of product formed.
To balance the chemical equation.
To calculate the theoretical yield.
To determine the limiting reactant.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If 2 moles of AlBr3 produce 3 moles of Br2, how many moles of Br2 are produced from 0.2 moles of AlBr3?
0.6
0.5
0.4
0.3
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of bromine used in the calculation?
27 g/mol
36 g/mol
18 g/mol
9 g/mol
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many grams of bromine are consumed to achieve a 66.9% yield?
27 g
18 g
45 g
36 g
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to convert moles to grams in chemical calculations?
To compare with experimental data.
To determine the purity of the reactants.
To simplify the calculation process.
To ensure accuracy in measurements.
Similar Resources on Wayground

6 questions
Molar Mass and Calculations Quiz
Interactive video
•
9th - 10th Grade

6 questions
Understanding Observations in Science
Interactive video
•
9th - 10th Grade

11 questions
Character Insights and Preferences in The Big Bang Theory
Interactive video
•
9th - 12th Grade

11 questions
Understanding Molecular and Empirical Formulas
Interactive video
•
9th - 12th Grade

6 questions
Understanding Markup Based on Cost
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

6 questions
FOREST Self-Discipline
Lesson
•
1st - 5th Grade

7 questions
Veteran's Day
Interactive video
•
3rd Grade

20 questions
Weekly Prefix check #2
Quiz
•
4th - 7th Grade
Discover more resources for Chemistry

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
2.6 Electron Configurations and Orbital Notations
Quiz
•
10th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Naming Ionic compounds
Quiz
•
10th Grade

14 questions
PERIODIC TRENDS
Quiz
•
11th Grade

16 questions
Naming Ionic Compounds
Quiz
•
9th - 11th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade