Naming and Formulas of Ionic Compounds
Interactive Video
•
Chemistry, Science
•
9th - 10th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary purpose of a chemical formula?
To provide a recipe for making a compound
To explain the history of a compound
To describe the taste of a compound
To list the colors of a compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In binary ionic compounds, what happens to the name of the non-metal?
It is written in uppercase
It is replaced with a number
It changes to an 'ide' ending
It keeps its original name
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the key characteristic of ionic compounds that the crisscross method helps to achieve?
The total charges must be zero
The total charges must be positive
The total charges must be equal to the atomic number
The total charges must be negative
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in the crisscross method?
Calculate the molecular weight
Write the elements with their charges in superscript
Draw a diagram of the compound
List the elements alphabetically
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When using the crisscross method, what should you do if the subscript is one?
Write it as zero
Ignore it
Double it
Underline it
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
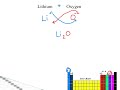
What is the correct chemical formula for a compound formed between lithium and oxygen?
LiO2
Li2O2
Li2O
LiO
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the name of the compound with the formula CaCl2?
Calcium chlorate
Calcium chlorite
Calcium dichloride
Calcium chloride
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
By signing up, you agree to our Terms of Service & Privacy Policy
Already have an account?
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade