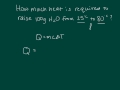
Heat Transfer and Temperature Change
Interactive Video
•
Physics
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary equation used to calculate the heat required to change the temperature of a substance?
P = IV
Q = mcΔT
E = mc^2
F = ma
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When considering a temperature change from 25°C to 80°C, what phase is water in?
Solid
Liquid
Gas
Plasma
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the specific heat capacity of water used in the example problem?
3.18 J/g°C
4.18 J/g°C
2.18 J/g°C
1.00 J/g°C
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you calculate the change in temperature (ΔT) in the equation Q = mcΔT?
Average of initial and final temperatures
Sum of initial and final temperatures
Final temperature minus initial temperature
Initial temperature minus final temperature
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the heat required to raise 100 grams of water from 25°C to 80°C?
229.9 J
2,299 J
22.99 J
22,990 J
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the context of the video, what does 'plug and chug' refer to?
Heating a substance to observe phase changes
Mixing substances to observe reactions
Substituting known values into an equation
Using a calculator to solve equations
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If the problem asked for the heat in kilojoules, what would be the answer?
229.9 kJ
22.99 kJ
2.299 kJ
0.2299 kJ
Create a free account and access millions of resources
Similar Resources on Wayground

11 questions
Energy Calculations and Phase Changes
Interactive video
•
9th - 10th Grade

11 questions
Understanding Specific Heat Capacity Concepts
Interactive video
•
9th - 10th Grade

11 questions
Calculating Energy in Phase Changes
Interactive video
•
9th - 10th Grade

11 questions
Specific Heat Capacity Concepts
Interactive video
•
9th - 10th Grade

11 questions
Thermodynamics and Phase Changes
Interactive video
•
9th - 10th Grade

11 questions
Phase Change and Heat Energy Concepts
Interactive video
•
9th - 10th Grade

11 questions
Specific Heat Capacity and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Heat Transfer and Specific Heat Capacity
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Physics

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade