Enthalpy Change and Hess's Law
Interactive Video
•
Chemistry
•
10th - 12th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary focus of Hess's Law?
Determining the enthalpy change of a reaction
Balancing chemical equations
Measuring the temperature change in a reaction
Calculating the rate of reaction
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does flipping a reaction affect its enthalpy change?
It doubles the enthalpy change
It has no effect on the enthalpy change
It halves the enthalpy change
It changes the sign of the enthalpy change
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the example problem, what is the first step in using thermochemical equations?
Cancel out unnecessary components
Calculate the total enthalpy change
Identify the given equations
Balance the chemical equation
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
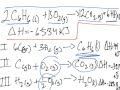
What is the enthalpy change for the combustion reaction of C6H6?
-6534.8 kJ
49 kJ
-393.5 kJ
-285.8 kJ
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which component is multiplied by 12 in the detailed solution?
CO2
Oxygen
Water
Graphite
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in calculating the enthalpy change of a reaction?
Multiplying the enthalpy change by a factor
Flipping the reaction
Adding all equations together
Balancing the chemical equation
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many equations are used in the example problem to find the enthalpy change?
Five
Four
Two
Three
Create a free account and access millions of resources
Similar Resources on Wayground

2 questions
Enthalpy Change in rate of H of a Reaction - Reaction Enthalpy
Interactive video
•
10th - 12th Grade

11 questions
Understanding Enthalpy and Thermochemistry
Interactive video
•
9th - 12th Grade

8 questions
Thermal Equations and Enthalpy Calculations
Interactive video
•
10th - 12th Grade

11 questions
Gibbs Free Energy and Thermodynamics Quiz
Interactive video
•
10th - 12th Grade

11 questions
Understanding Hess's Law and Enthalpy Changes
Interactive video
•
10th - 12th Grade

11 questions
Thermochemical Equations and Properties
Interactive video
•
9th - 12th Grade

11 questions
Thermochemistry Concepts and Applications
Interactive video
•
9th - 12th Grade

11 questions
Thermochemistry Concepts and Calculations
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

20 questions
Metric Conversions
Quiz
•
11th Grade