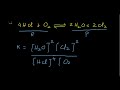
Chemical Reactions and Products
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the product formed when diatomic oxygen is converted during a lightning storm?
Ozone
Nitrogen
Carbon Dioxide
Water
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the reaction of HCl with oxygen, what are the products formed?
Methane and Water
Carbon Dioxide and Water
Hydrogen and Oxygen
Water and Chlorine
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of water are produced in the reaction between HCl and oxygen?
1
2
4
3
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What are the reactants in the reaction forming ethyl acetate?
Acetic Acid and Ethanol
Acetic Acid and Water
Water and Ethanol
Methane and Water
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the decomposition of hydrogen fluoride, what gases are produced?
Hydrogen and Fluorine
Oxygen and Nitrogen
Chlorine and Oxygen
Carbon Dioxide and Water
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many moles of hydrogen fluoride decompose in the given reaction?
3
4
1
2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the product formed from nitrogen dioxide in the given reaction?
Methane
Ozone
Water
Dinitrogen Tetroxide
Create a free account and access millions of resources
Similar Resources on Wayground

6 questions
Limiting Reactants in Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Baking Soda and Vinegar Reactions
Interactive video
•
9th - 10th Grade

11 questions
Mastering Mole Ratios in Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Rocket Propellant Chemistry Quiz
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry and Molar Conversions Quiz
Interactive video
•
9th - 10th Grade

11 questions
Stoichiometry Concepts and Calculations
Interactive video
•
9th - 10th Grade

11 questions
Mass-Mole Stoichiometry Quiz
Interactive video
•
9th - 10th Grade

11 questions
Titration Calculations and Concepts
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade