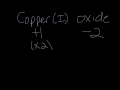
Oxidation States and Chemical Formulas
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the main focus of the video tutorial?
Drawing ionic compounds from their names
Balancing chemical equations
Understanding covalent bonds
Learning about noble gases
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation state of magnesium in magnesium sulfide?
+1
+2
-1
-2
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many sodium atoms are needed to balance the charge in sodium oxide?
Three
Two
One
Four
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation state of copper in copper(I) oxide?
+4
+2
+1
+3
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many copper atoms are required to balance the charge in copper(I) oxide?
Four
One
Two
Three
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the oxidation state of aluminum in aluminum nitrate?
+3
+2
+1
+4
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formula for nitrate ion?
NO2-
NO3-
SO4-
NH4+
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Iron(III) Nitrate and Ionic Compounds
Interactive video
•
9th - 10th Grade

9 questions
Understanding Vanadium and Carbonate Compounds
Interactive video
•
9th - 10th Grade

11 questions
Copper(II) Nitrate and Its Properties
Interactive video
•
9th - 10th Grade

8 questions
Understanding Silver Compounds and Ions
Interactive video
•
9th - 10th Grade

8 questions
Naming and Charges of Copper Compounds
Interactive video
•
9th - 10th Grade

7 questions
Copper Compounds and Hydroxide Ions
Interactive video
•
9th - 10th Grade

11 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade

9 questions
Ionic Compounds and Nomenclature
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab safety
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

12 questions
Counting Significant Figures Quick Check
Quiz
•
9th - 12th Grade

10 questions
Significant Figures Int 2
Quiz
•
9th - 12th Grade

19 questions
States of Matter Review
Quiz
•
10th Grade

21 questions
Lab Safety
Quiz
•
10th Grade