
Thermodynamics and Phase Changes
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Patricia Brown
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the process called when a solid turns into a liquid?
Sublimation
Evaporation
Condensation
Melting
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
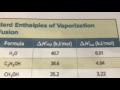
How much energy is required to melt one mole of ice?
10.5 kJ/mol
6.01 kJ/mol
40.7 kJ/mol
15.2 kJ/mol
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the term for the transition from liquid to gas?
Condensation
Fusion
Evaporation
Freezing
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How much energy is needed to vaporize one mole of water?
6.01 kJ/mol
20.5 kJ/mol
40.7 kJ/mol
15.2 kJ/mol
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to energy during condensation?
Energy is released
Energy is absorbed
Energy remains constant
Energy is doubled
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is an exothermic process?
Sublimation
Condensation
Melting
Evaporation
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does an endothermic reaction involve?
No heat change
Absorption of heat
Release of heat
Doubling of heat
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

8 questions
Menulis rumus kimia sederhana
Interactive video
•
10th Grade

11 questions
Alkaline Earth Metals
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Bling bling revelations hit powerful French union chief
Interactive video
•
9th - 10th Grade

6 questions
David Young - Global Education for All
Interactive video
•
9th - 10th Grade

6 questions
What Is the Water Cycle?: Condensation
Interactive video
•
10th - 12th Grade

6 questions
Cambios físicos: recapitulando
Interactive video
•
10th - 12th Grade

6 questions
CLEAN : PROFILE: American director Steven Spielberg
Interactive video
•
9th - 12th Grade

6 questions
Interview with Labour leader Sir Keir Starmer on Afghan airlift
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

10 questions
Probability Practice
Quiz
•
4th Grade

15 questions
Probability on Number LIne
Quiz
•
4th Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

6 questions
Appropriate Chromebook Usage
Lesson
•
7th Grade

10 questions
Greek Bases tele and phon
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Predicting Products
Quiz
•
9th - 12th Grade

11 questions
Balancing Chemical Equations
Lesson
•
9th Grade

10 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

19 questions
Stoichiometry, % yield, Limiting Reactants
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

10 questions
Exploring Ionic and Covalent Bonding Concepts
Interactive video
•
6th - 10th Grade

7 questions
GCSE Chemistry - Balancing Chemical Equations #4
Interactive video
•
9th - 10th Grade

12 questions
Percent Yield
Quiz
•
10th Grade