
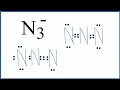
Lewis Structures and N3- Ion
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in the N3- ion?
14
17
15
16
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How are the nitrogen atoms arranged in the initial Lewis structure of N3-?
In a triangle
In a square
In a row
In a circle
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of moving electrons from the outside to the inside in the Lewis structure?
To change the element
To decrease the total valence electrons
To form double or triple bonds
To increase the number of atoms
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is a valid alternative Lewis structure for N3-?
Two single bonds
Three single bonds
One triple bond and one single bond
Two triple bonds
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the formal charge on the central nitrogen in the alternative Lewis structure with a triple bond?
0
+1
-1
-2
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is the Lewis structure with two double bonds considered better?
It has formal charges closer to zero
It is more symmetrical
It has more atoms
It uses fewer electrons
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total charge on the N3- ion?
-2
+1
0
-1
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Igneous and Metamorphic Rocks: Contact Metamorphism
Interactive video
•
10th - 12th Grade

8 questions
Menulis rumus kimia sederhana
Interactive video
•
10th Grade

8 questions
Dianne Bilyak "A Bell of Water, Ringing"
Interactive video
•
9th - 10th Grade

6 questions
Follies in Sammy's
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : New Mali junta opens talks on transition to civilian rule
Interactive video
•
9th - 10th Grade

6 questions
I WONDER - Are There Different Parts To The Intestine? Me Pregunto - Existen Diferentes Partes En Los Intestinos?
Interactive video
•
KG - 12th Grade

6 questions
CLEAN : Brett Ratner gets star on the Hollywood Walk of Fame
Interactive video
•
9th - 12th Grade

6 questions
Die Geschichte vom Suppen-Kaspar - Märchen - Deutsch lernen
Interactive video
•
10th - 12th Grade
Popular Resources on Wayground

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

29 questions
Alg. 1 Section 5.1 Coordinate Plane
Quiz
•
9th Grade

22 questions
fractions
Quiz
•
3rd Grade

11 questions
FOREST Effective communication
Lesson
•
KG

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

22 questions
Unit 9 Gas Law Quiz
Quiz
•
10th Grade

10 questions
Exploring Types of Chemical Reactions
Interactive video
•
6th - 10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

20 questions
Acids and Bases
Quiz
•
10th Grade

30 questions
Energy Review
Quiz
•
9th Grade

7 questions
GCSE Chemistry - Balancing Chemical Equations #4
Interactive video
•
9th - 10th Grade

20 questions
Chemistry: Classification of Matter
Quiz
•
10th Grade

40 questions
Unit 3 (Part 1) Chemical Equations & Reactions Review Game
Quiz
•
8th - 12th Grade