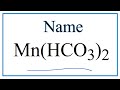
Naming and Charges of Polyatomic Ions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Sophia Harris
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of metal is manganese in the compound MnH(CO3)2?
Transition metal
Alkaline earth metal
Noble gas
Alkali metal
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in naming the compound MnH(CO3)2?
Calculate the net charge
Write the name of the metal as it appears on the periodic table
Determine the charge of the metal
Identify the polyatomic ion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the polyatomic ion present in MnH(CO3)2?
Nitrate
Bicarbonate
Sulfate
Carbonate
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is another name for the bicarbonate ion?
Sulfate ion
Hydrogen carbonate ion
Carbonate ion
Nitrate ion
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it necessary to indicate the charge of manganese in the compound name?
Because manganese is a transition metal
Because manganese is a non-metal
To differentiate it from other metals
To indicate the number of polyatomic ions
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the bicarbonate ion in MnH(CO3)2?
1+
2+
1-
2-
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you represent the charge of manganese in the compound name?
Using a subscript
With a suffix
With a prefix
By a Roman numeral in parentheses
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final name of the compound MnH(CO3)2?
Manganese(III) bicarbonate
Manganese(IV) bicarbonate
Manganese(II) bicarbonate
Manganese bicarbonate
9.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the Roman numeral in the compound name indicate?
The total charge of the compound
The number of polyatomic ions
The charge of the transition metal
The number of hydrogen atoms
Similar Resources on Wayground

8 questions
Ionic Compounds and Naming Conventions
Interactive video
•
9th - 10th Grade

6 questions
Understanding Elements in the Periodic Table
Interactive video
•
9th - 10th Grade

6 questions
Understanding Roman Numerals in Ionic Compounds
Interactive video
•
9th - 10th Grade

6 questions
Sodium Ion vs Lithium Ion Batteries Quiz
Interactive video
•
9th - 10th Grade

6 questions
Nitrate Ion Test Quiz
Interactive video
•
9th - 10th Grade

6 questions
Understanding Ionic Bonds and Dot and Cross Diagrams
Interactive video
•
9th - 10th Grade

6 questions
Chemical Compounds Quiz
Interactive video
•
9th - 10th Grade

6 questions
Polarity and Geometry of NO2+
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

6 questions
FOREST Self-Discipline
Lesson
•
1st - 5th Grade

7 questions
Veteran's Day
Interactive video
•
3rd Grade

20 questions
Weekly Prefix check #2
Quiz
•
4th - 7th Grade
Discover more resources for Chemistry

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
2.6 Electron Configurations and Orbital Notations
Quiz
•
10th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Naming Ionic compounds
Quiz
•
10th Grade

16 questions
Naming Ionic Compounds
Quiz
•
9th - 11th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

61 questions
Honors Periodic Table Unit Review
Quiz
•
9th - 11th Grade