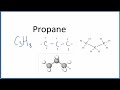
Understanding Propane and Hexane Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Olivia Brooks
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the molecular formula C3H8 indicate about propane?
It has three hydrogen and eight carbon atoms.
It has three carbon and eight hydrogen atoms.
It has eight carbon and three hydrogen atoms.
It has eight hydrogen and three oxygen atoms.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bonds are present in propane?
Single bonds between carbon and hydrogen atoms
Triple bonds between carbon atoms
Ionic bonds between carbon and hydrogen atoms
Double bonds between carbon atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How are the carbon atoms arranged in the structural formula of propane?
In a straight line with double bonds
In a circular shape with single bonds
In a straight line with single bonds
In a triangular shape with single bonds
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the three-dimensional representation of propane?
It shows the weight of the molecule.
It shows the temperature of the molecule.
It shows the bond angles between atoms.
It shows the color of the atoms.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the molecular model of propane, what do the white spheres represent?
Nitrogen atoms
Oxygen atoms
Carbon atoms
Hydrogen atoms
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is omitted in the skeletal structure of propane?
Nitrogen atoms
Carbon atoms
Hydrogen atoms
Oxygen atoms
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How does the skeletal structure of propane differ from its structural formula?
It includes double bonds.
It omits the hydrogen atoms.
It omits the carbon atoms.
It includes all hydrogen atoms.
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
SKorean president meets Japanese PM
Interactive video
•
9th - 10th Grade

8 questions
Lone Pairs and Molecular Geometry
Interactive video
•
9th - 10th Grade

8 questions
Moles to Liters Conversion Concepts
Interactive video
•
9th - 10th Grade

10 questions
Hope Diamond Analysis and Significance
Interactive video
•
9th - 10th Grade

11 questions
Moles and Molecular Formulas
Interactive video
•
9th - 10th Grade

11 questions
Hexane Molecular Structure and Properties
Interactive video
•
9th - 10th Grade

8 questions
What is the recursive formula and how do we use it
Interactive video
•
9th - 10th Grade

8 questions
CLEAN : Tara research schooner returns after testing ocean plankton and pollution
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

10 questions
Formative 3BD: Ionic Bonds
Quiz
•
9th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade