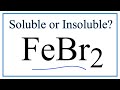
Iron(II) Bromide and Solubility
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for iron(II) bromide?
Fe2Br
Fe2Br3
FeBr2
FeBr3
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, what is the expected solubility of FeBr2 in water?
Insoluble
Not enough information
Slightly soluble
Soluble
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility table indicate about the solubility of iron(II) bromide?
It is insoluble
It is soluble
It is not listed
It is slightly soluble
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where can you find the complete solubility table mentioned in the video?
In the appendix
On the blackboard
In the textbook
In the video description
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are formed when FeBr2 dissolves in water?
Fe3+ and Br2
Fe2+ and Br2
Fe2+ and Br-
Fe3+ and Br-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bromide ions are produced for each iron(II) ion when FeBr2 dissolves?
Two
Four
Three
One
Similar Resources on Wayground

7 questions
Solubility and Properties of NaHCO3
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Magnesium Phosphate
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Barium Sulfate
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Silver Compounds
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Lithium Bromide
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Phosphates in Water
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Manganese II Chloride
Interactive video
•
9th - 10th Grade

9 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

15 questions
Core 4 of Customer Service - Student Edition
Quiz
•
6th - 8th Grade

15 questions
What is Bullying?- Bullying Lesson Series 6-12
Lesson
•
11th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Atomic Structure
Quiz
•
10th Grade

35 questions
Electron Configuration
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

21 questions
Naming Covalent Compounds
Lesson
•
10th Grade

15 questions
Atomic Structure
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

14 questions
Molecules, Compounds, & Elements
Quiz
•
9th Grade