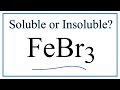
Chemical Properties of Iron Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Sophia Harris
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical name of FeBr3?
Iron(III) bromide
Iron(III) chloride
Iron(II) bromide
Iron(II) chloride
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which ion generally makes compounds soluble?
Nitrate
Bromide
Sulfate
Chloride
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What tool can be used to confirm the solubility of a compound?
Periodic table
Solubility table
Molecular weight chart
pH scale
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the solubility table, what does the symbol 'S' indicate?
Solid
Suspended
Soluble
Saturated
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
When FeBr3 dissolves in water, into which ions does it dissociate?
Fe2+ and Br2-
Fe3+ and Br2-
Fe3+ and Br-
Fe2+ and Br-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many bromide ions are produced for each iron(III) ion when FeBr3 dissolves?
Three
Two
One
Four
Similar Resources on Wayground

6 questions
Understanding Hydrogen Reactions
Interactive video
•
9th - 10th Grade

8 questions
Molar Mass and Conversion Concepts
Interactive video
•
9th - 10th Grade

9 questions
Solubility of Magnesium Hydroxide
Interactive video
•
9th - 10th Grade

6 questions
Origins of Earth's Water
Interactive video
•
9th - 10th Grade

6 questions
Igneous Rocks and Their Formations
Interactive video
•
9th - 10th Grade

6 questions
Meteor Strike over Russia Quiz
Interactive video
•
9th - 10th Grade

6 questions
The Menorah: History and Mysteries
Interactive video
•
9th - 10th Grade

6 questions
Epic Crossover Quiz
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Forest Self-Management
Lesson
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

30 questions
Thanksgiving Trivia
Quiz
•
9th - 12th Grade

30 questions
Thanksgiving Trivia
Quiz
•
6th Grade

11 questions
Would You Rather - Thanksgiving
Lesson
•
KG - 12th Grade

48 questions
The Eagle Way
Quiz
•
6th Grade

10 questions
Identifying equations
Quiz
•
KG - University

10 questions
Thanksgiving
Lesson
•
5th - 7th Grade
Discover more resources for Chemistry

88 questions
Test Review
Quiz
•
9th Grade

20 questions
Unit 3, Quiz #6 Practice - Types of Covalent
Quiz
•
9th - 12th Grade

22 questions
Unit 2 Part 1 Rumble
Quiz
•
10th Grade

20 questions
Molar Mass
Quiz
•
9th - 12th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Electron Configuration
Quiz
•
10th - 12th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade