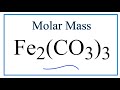
Molar Mass and Chemical Calculations
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Mia Campbell
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of iron (Fe) as given in the video?
55.85 g/mol
12.01 g/mol
16.00 g/mol
291.73 g/mol
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbon atoms are present in the formula Fe2(CO3)3?
6
3
2
1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of oxygen (O) as used in the calculation?
291.73 g/mol
55.85 g/mol
12.01 g/mol
16.00 g/mol
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why do we multiply the entire formula by three in the calculation?
Because there are three carbonate ions
Because there are three oxygen atoms
Because there are three iron atoms
Because there are three carbon atoms
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final calculated molar mass of iron(III) carbonate?
55.85 g/mol
12.01 g/mol
16.00 g/mol
291.73 g/mol
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What should you consider about your periodic table when calculating molar mass?
The size of the table
The rounding of decimal places
The number of elements
The color of the table
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
If your periodic table rounds to more decimal places, what might happen to your answer?
It will be exactly the same
It will be significantly different
It will be incorrect
It might be slightly different
Similar Resources on Wayground

6 questions
Three hundred prisoners take seven guards hostage in riot at Menard State Prison in Chester, Illinois
Interactive video
•
9th - 10th Grade

11 questions
Perpetual Motion Machines and Thermodynamics
Interactive video
•
9th - 12th Grade

6 questions
CLEAN : Hungary declares state of crisis after sealing border
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Entertaining Kiev's protest square, a full
Interactive video
•
9th - 10th Grade

2 questions
Soviet made propaganda film of Brezhnev's visit to Cuba
Interactive video
•
9th - 10th Grade

2 questions
CLEAN : Nigerians push for clarity on absentee president's health
Interactive video
•
9th - 10th Grade

6 questions
The Modernization Theory Explained | The Stages Of Economic Growth
Interactive video
•
KG - University

6 questions
GE dedicates Jet Production and Development Center in Lockland, OH and Boeing B-47 under construction in Wichita, KS
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade