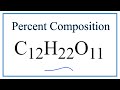
Molar Mass and Composition of Sucrose
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Emma Peterson
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula of sucrose?
C6H12O6
C12H22O11
C2H5OH
CH4
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to calculate the molar mass of sucrose?
To calculate the density
To find the percent composition by mass
To determine the number of molecules
To measure the volume
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molar mass of sucrose?
180.18 g/mol
342.34 g/mol
98.08 g/mol
58.44 g/mol
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many carbon atoms are present in a molecule of sucrose?
12
11
6
22
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent composition of carbon in sucrose?
6.49%
51.41%
12.01%
42.10%
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many hydrogen atoms are present in a molecule of sucrose?
22
12
11
6
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the percent composition of hydrogen in sucrose?
51.41%
6.49%
42.10%
1.01%
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Percent Composition and Molar Mass
Interactive video
•
9th - 10th Grade

9 questions
Percent Composition and Molar Mass of NiCl2
Interactive video
•
9th - 10th Grade

9 questions
Calcium Nitrate and Water Composition
Interactive video
•
9th - 10th Grade

6 questions
Understanding Carbohydrates
Interactive video
•
9th - 10th Grade

11 questions
Percent Composition and Molar Mass Calculations
Interactive video
•
9th - 10th Grade

11 questions
Percent Composition of HCl
Interactive video
•
9th - 10th Grade

6 questions
Glucose and Water Interactions
Interactive video
•
9th - 10th Grade

11 questions
Empirical Formulas and Calculations
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
9/11 Experience and Reflections
Interactive video
•
10th - 12th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

9 questions
Tips & Tricks
Lesson
•
6th - 8th Grade
Discover more resources for Chemistry

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

15 questions
Significant figures and Measurements
Quiz
•
10th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

30 questions
Unit 1.2 Nuclear Chemistry
Quiz
•
10th Grade