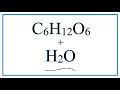
Glucose and Water Interactions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for glucose?
C6H12O6
H2O
C12H22O11
NaCl
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What state is glucose in before it is dissolved in water?
Liquid
Aqueous
Solid
Gas
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to glucose when it is added to water?
It evaporates into the air.
It reacts with water to form a new compound.
It dissolves in water without any chemical reaction.
It remains a solid and does not dissolve.
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'aq' notation signify in a chemical equation?
The substance is a liquid.
The substance is a solid.
The substance is a gas.
The substance is dissolved in water.
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is water not included on the right side of the equation after glucose dissolves?
Because water evaporates.
Because water reacts and forms a new compound.
Because water is not needed for the dissolution process.
Because the 'aq' notation implies the presence of water.
Similar Resources on Wayground

8 questions
Sodium Bromide and Water Reactions
Interactive video
•
9th - 10th Grade

9 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

7 questions
Solubility and Ions of Sodium Perchlorate
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Ammonium Compounds
Interactive video
•
9th - 10th Grade

10 questions
Zinc Nitrate and Aqueous Solutions
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Chemical Reactions
Interactive video
•
9th - 10th Grade

8 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

6 questions
Ionic Compounds and Their Properties
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

10 questions
Chaffey
Quiz
•
9th - 12th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

22 questions
6-8 Digital Citizenship Review
Quiz
•
6th - 8th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab safety
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

12 questions
Counting Significant Figures Quick Check
Quiz
•
9th - 12th Grade

10 questions
Significant Figures Int 2
Quiz
•
9th - 12th Grade

19 questions
States of Matter Review
Quiz
•
10th Grade

21 questions
Lab Safety
Quiz
•
10th Grade