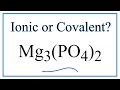
Magnesium Phosphate Bonding and Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Mia Campbell
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is magnesium phosphate primarily classified as?
Metallic
Molecular
Ionic
Covalent
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which elements in magnesium phosphate are considered non-metals?
Magnesium and Phosphate
Phosphate and Oxygen
Magnesium and Oxygen
Phosphorus and Magnesium
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the ionic charge of a magnesium ion in magnesium phosphate?
3+
2+
1+
0
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many phosphate ions are present in magnesium phosphate?
Four
Three
Two
One
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total negative charge contributed by the phosphate ions in magnesium phosphate?
9-
12-
6-
3-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the net charge of magnesium phosphate?
Zero
Positive
Negative
Variable
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of bond is formed between magnesium and phosphate ions?
Covalent
Ionic
Metallic
Hydrogen
Create a free account and access millions of resources
Similar Resources on Wayground

7 questions
Phosphate Ion Properties and Characteristics
Interactive video
•
9th - 10th Grade

9 questions
Hydroxide Ion and Magnesium Compounds
Interactive video
•
9th - 10th Grade

10 questions
Nickel Compounds and Phosphate Ions
Interactive video
•
9th - 10th Grade

11 questions
Ionic Compounds and Bonding Concepts
Interactive video
•
9th - 10th Grade

10 questions
Electronegativity and Ionic Compounds
Interactive video
•
9th - 10th Grade

7 questions
Barium Carbonate and Related Concepts
Interactive video
•
9th - 10th Grade

11 questions
Single Replacement Reactions in Chemistry
Interactive video
•
9th - 10th Grade

10 questions
Magnesium Nitrate and Ionic Bonds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
SR&R 2025-2026 Practice Quiz
Quiz
•
6th - 8th Grade

30 questions
Review of Grade Level Rules WJH
Quiz
•
6th - 8th Grade

6 questions
PRIDE in the Hallways and Bathrooms
Lesson
•
12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

15 questions
Subtracting Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

10 questions
Unit 1b Lesson 1 Quick Check
Quiz
•
9th Grade

12 questions
significant figures and calculations
Quiz
•
10th - 12th Grade

20 questions
12.2 Scientific Notation and Significant Figures
Quiz
•
10th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade

12 questions
Atomic Structure and isotopes
Quiz
•
10th Grade