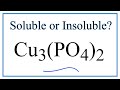
Solubility of Copper II Phosphate
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
7 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of copper II phosphate in water?
Soluble
Insoluble
Partially soluble
Highly soluble
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is primarily responsible for the insolubility of copper II phosphate?
Cl-
Cu2+
PO4 3-
Na+
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to solubility rules, which of the following is generally insoluble?
Chlorides
Phosphates
Sulfates
Nitrates
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'i' symbol indicate on a solubility chart?
Partially soluble
Insoluble
Soluble
Highly soluble
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How can the solubility of copper II phosphate be confirmed using a chart?
By checking the solubility of Cu2+ with PO4 3-
By looking for a 's' symbol
By finding the solubility of Na+ with Cl-
By checking the solubility of Cu2+ with Cl-
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens when copper II phosphate is placed in water?
It remains mostly undissolved
It dissolves partially
It dissolves completely
It reacts violently
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the significance of the small amount of copper II phosphate that dissolves in water?
It indicates high solubility
It suggests a chemical reaction
It is negligible and considered insoluble
It shows partial solubility
Similar Resources on Wayground

6 questions
Net Ionic Equations and Solubility
Interactive video
•
9th - 10th Grade

9 questions
Solubility of Potassium Phosphate
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Hydroxides and Ions
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Lithium Bromide
Interactive video
•
9th - 10th Grade

6 questions
Strontium Nitrate and Ion Properties
Interactive video
•
9th - 10th Grade

6 questions
Aluminum Oxide Solubility Concepts
Interactive video
•
9th - 10th Grade

6 questions
Solubility of Phosphates in Water
Interactive video
•
9th - 10th Grade

8 questions
Solubility of Magnesium Phosphate
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

50 questions
Trivia 7/25
Quiz
•
12th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

11 questions
Negative Exponents
Quiz
•
7th - 8th Grade

12 questions
Exponent Expressions
Quiz
•
6th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

20 questions
One Step Equations All Operations
Quiz
•
6th - 7th Grade

18 questions
"A Quilt of a Country"
Quiz
•
9th Grade