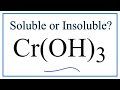
Solubility of Hydroxides and Ions
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of chromium-3 hydroxide in water?
Insoluble
Soluble
Highly soluble
Partially soluble
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which group of elements is generally very soluble in water?
Noble gases
Group 2 elements
Transition metals
Group 1 elements
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the solubility status of most hydroxide salts?
Insoluble
Highly soluble
Slightly soluble
Completely insoluble
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ion is mentioned as being very soluble in water?
Ammonium ion
Nitrate ion
Carbonate ion
Sulfate ion
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the experimental data confirm about chromium-3 hydroxide?
It is insoluble
It is reactive with water
It is highly soluble
It is slightly soluble
Similar Resources on Wayground

6 questions
Manganese(IV) Bromide Chemistry Concepts
Interactive video
•
9th - 10th Grade

6 questions
Chemical Reactions and Products
Interactive video
•
9th - 10th Grade

6 questions
Oxidation Numbers and Hydroxide Ion
Interactive video
•
9th - 10th Grade

6 questions
Ammonium Chlorate and Polyatomic Ions
Interactive video
•
9th - 10th Grade

7 questions
Understanding Potassium Chromate and Ions
Interactive video
•
9th - 10th Grade

7 questions
Dissolution of KMnO4 in Water
Interactive video
•
9th - 10th Grade

7 questions
Ionic Charges and Chemical Formulas
Interactive video
•
9th - 10th Grade

6 questions
Chemical Equation Balancing and Atoms
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

20 questions
Brand Labels
Quiz
•
5th - 12th Grade

11 questions
NEASC Extended Advisory
Lesson
•
9th - 12th Grade

10 questions
Ice Breaker Trivia: Food from Around the World
Quiz
•
3rd - 12th Grade

10 questions
Boomer ⚡ Zoomer - Holiday Movies
Quiz
•
KG - University

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

10 questions
Multiplication and Division Unknowns
Quiz
•
3rd Grade

20 questions
Multiplying and Dividing Integers
Quiz
•
7th Grade
Discover more resources for Chemistry

32 questions
Unit 2/3 Test Electrons & Periodic Table
Quiz
•
10th Grade

20 questions
Physical or Chemical Change/Phases
Quiz
•
8th Grade - University

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

33 questions
Unit 2-3 Electrons and Periodic Trends
Quiz
•
10th Grade

21 questions
Isotopes and Ions
Quiz
•
9th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

20 questions
electron configurations and orbital notation
Quiz
•
9th - 12th Grade