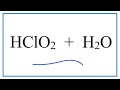
Understanding Aqueous Ions and Acids
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the primary indication that HClO2 is an acid?
The presence of H at the front
The presence of ClO2
Its color
Its reaction with metals
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is chlorous acid considered a weak acid?
It is not on the list of strong acids
It reacts violently with water
It completely dissociates in water
It has a high pH
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to chlorous acid in water?
It fully dissociates into ions
It partially dissociates into ions
It remains completely undissociated
It forms a solid precipitate
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the equilibrium arrow in the reaction indicate?
The reaction is exothermic
The reaction can proceed in both directions
The reaction is irreversible
The reaction goes to completion
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'aq' signify when written after ions?
The ions are in a liquid state
The ions are in a solid state
The ions are dissolved in water
The ions are in a gaseous state
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to note the aqueous state of ions?
It indicates the ions are reactive
It suggests the ions are volatile
It shows the ions are in a solution
It means the ions are stable
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is formed when H+ ions join with water molecules?
Oxygen molecules
Chloride ions
Hydronium ions
Hydroxide ions
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Ions and Their Reactions
Interactive video
•
9th - 10th Grade

10 questions
Understanding the Hydronium Ion
Interactive video
•
9th - 10th Grade

11 questions
Acids, Bases, and Their Reactions
Interactive video
•
9th - 10th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
9th - 10th Grade

11 questions
Hydronium Ion and Acid Properties
Interactive video
•
9th - 10th Grade

9 questions
Formal Charge and Valence Electrons
Interactive video
•
9th - 10th Grade

11 questions
Acids, Bases, and Their Concepts
Interactive video
•
9th - 10th Grade

11 questions
Acid-Base Chemistry Concepts
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Appointment Passes Review
Quiz
•
6th - 8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
Grammar Review
Quiz
•
6th - 9th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

12 questions
Significant figures
Quiz
•
9th - 12th Grade

30 questions
Aca Nuclear Chemistry
Quiz
•
10th Grade

16 questions
Counting Sig Figs
Quiz
•
10th - 12th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
Significant Figures
Quiz
•
10th - 11th Grade