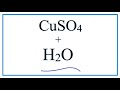
Copper II Sulfate and Its Properties
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Lucas Foster
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the chemical formula for anhydrous copper II sulfate?
CuSO4
CuSO4·5H2O
Cu2SO4
CuSO3
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'penta' in copper II sulfate pentahydrate indicate?
Five oxygen atoms
Five water molecules
Five sulfate ions
Five copper atoms
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to copper II sulfate pentahydrate when it is heated?
It forms a gas
It dissolves in water
It becomes anhydrous
It turns blue
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the state of copper II sulfate pentahydrate when it is added to water?
Plasma
Gas
Liquid
Solid
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What charge does the sulfate ion carry?
2-
1-
1+
2+
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'aqueous' mean in a chemical equation?
Solid state
Gaseous state
Liquid state
Dissolved in water
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are formed when copper II sulfate pentahydrate dissolves in water?
Cu2+ and SO4 2-
Cu+ and SO3 2-
Cu+ and SO4 2-
Cu2+ and SO3 2-
Create a free account and access millions of resources
Similar Resources on Wayground

8 questions
Copper(II) Sulfate and Its Properties
Interactive video
•
9th - 10th Grade

10 questions
Copper II Sulfate Pentahydrate Concepts
Interactive video
•
9th - 10th Grade

8 questions
Copper(II) Sulfate Properties and Reactions
Interactive video
•
9th - 10th Grade

6 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

10 questions
Hydrated Iron(II) Sulfate Concepts
Interactive video
•
9th - 10th Grade

10 questions
pH and Properties of CuSO4 Solutions
Interactive video
•
9th - 10th Grade

9 questions
Net Ionic Equations and Spectator Ions
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

55 questions
CHS Student Handbook 25-26
Quiz
•
9th Grade

10 questions
Afterschool Activities & Sports
Quiz
•
6th - 8th Grade

15 questions
PRIDE
Quiz
•
6th - 8th Grade

15 questions
Cool Tool:Chromebook
Quiz
•
6th - 8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

20 questions
Bullying
Quiz
•
7th Grade

18 questions
7SS - 30a - Budgeting
Quiz
•
6th - 8th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

15 questions
Atoms, Ions, and Isotopes
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
Lab Safety
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade

40 questions
Lab Safety
Quiz
•
9th - 12th Grade