
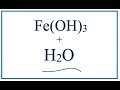
Iron(III) Hydroxide Solubility Concepts
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Lucas Foster
FREE Resource
Read more
9 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the expected outcome when iron(III) hydroxide is mixed with water?
It dissolves completely.
It forms a gas.
It reacts violently.
It dissociates into ions.
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What ions are expected to form if iron(III) hydroxide dissociates in water?
Fe2+ and OH-
Fe3+ and OH-
Fe2+ and H+
Fe3+ and O2-
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of the hydroxide ion formed during dissociation?
Variable
Positive
Negative
Neutral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What coefficient is needed in front of OH- to balance the dissociation equation of iron(III) hydroxide?
4
3
1
2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to check a solubility table when predicting the behavior of iron(III) hydroxide in water?
To confirm the color change.
To determine the reaction speed.
To verify its solubility.
To measure the temperature change.
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the solubility table indicate about iron(III) hydroxide?
It is insoluble.
It is slightly soluble.
It forms a precipitate.
It is highly soluble.
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'I' symbol in the solubility table stand for?
Indeterminate
Ionic
Insoluble
Intense
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
VOICED : Cyprus struggles to manage waste as tourist numbers soar
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Fearful Hong Kongers hold limited British passports as sign of hope
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Hundreds queue for Olympic mascot merch at flagship Beijing store
Interactive video
•
9th - 12th Grade

6 questions
Mixing Substances
Interactive video
•
10th - 12th Grade

11 questions
Ancient Greeks and Other Civilizations
Interactive video
•
9th - 12th Grade

6 questions
VOICED : Polvo del Sahara afecta las playas mexicanas
Interactive video
•
9th - 10th Grade

6 questions
Equilibrium Constant Expression
Interactive video
•
10th Grade - University

8 questions
Menulis rumus kimia sederhana
Interactive video
•
10th Grade
Popular Resources on Wayground

7 questions
History of Valentine's Day
Interactive video
•
4th Grade

15 questions
Fractions on a Number Line
Quiz
•
3rd Grade

20 questions
Equivalent Fractions
Quiz
•
3rd Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

22 questions
fractions
Quiz
•
3rd Grade

15 questions
Valentine's Day Trivia
Quiz
•
3rd Grade

20 questions
Main Idea and Details
Quiz
•
5th Grade

20 questions
Context Clues
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 8 Stoichiometry Review
Quiz
•
10th Grade

20 questions
Types of Chemical Reactions
Quiz
•
9th - 12th Grade

19 questions
Stoichiometry, Limiting Reactants, and Percent Yield
Quiz
•
10th Grade

10 questions
Formative 3BD: Ionic Bonds
Quiz
•
9th Grade

15 questions
Balancing Chemical Equations
Quiz
•
10th - 12th Grade

20 questions
Naming & Writing Chemical Formulas
Quiz
•
10th Grade

10 questions
Identifying types of reactions
Quiz
•
9th - 12th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade