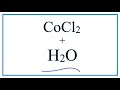
Chemical Reactions and Ionic Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Jackson Turner
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is formed when a metal reacts with a non-metal?
Organic compound
Metallic compound
Covalent compound
Ionic compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What happens to cobalt(II) chloride when it is added to water?
It forms a precipitate
It reacts to form a gas
It remains unchanged
It dissolves and dissociates into ions
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which ions are produced when cobalt(II) chloride dissociates in water?
Co2+ and Cl-
Co3+ and Cl2-
Co+ and Cl2-
Co3+ and Cl-
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is a coefficient of two placed in front of the chloride ion in the dissociation equation?
To represent a solid state
To show the reaction is reversible
To indicate the presence of two chloride ions
To balance the charge
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the notation 'aq' signify in a chemical equation?
The substance is a liquid
The substance is dissolved in water
The substance is a gas
The substance is a solid
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is water not written on the product side of the equation for the reaction of cobalt(II) chloride with water?
Because it is not involved in the reaction
Because it is a reactant
Because the ions are already in aqueous form
Because it evaporates
Similar Resources on Wayground

6 questions
Barium and Chlorine Compounds
Interactive video
•
9th - 10th Grade

6 questions
Iron II Chloride Concepts
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Reactions Techniques
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Copper Chemistry
Interactive video
•
9th - 10th Grade

7 questions
Balancing Chemical Equations Concepts
Interactive video
•
9th - 10th Grade

7 questions
Chemical Reactions and Equations
Interactive video
•
9th - 10th Grade

8 questions
Rubidium Chloride and Water Chemistry
Interactive video
•
9th - 10th Grade

8 questions
Titanium and Chloride Compounds
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Video Games
Quiz
•
6th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
UPDATED FOREST Kindness 9-22
Lesson
•
9th - 12th Grade

22 questions
Adding Integers
Quiz
•
6th Grade

15 questions
Subtracting Integers
Quiz
•
7th Grade

20 questions
US Constitution Quiz
Quiz
•
11th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

15 questions
Isotopes/structure of an atom
Quiz
•
10th Grade

20 questions
Atomic Structure
Quiz
•
10th - 12th Grade

20 questions
COUNTING ATOMS
Quiz
•
10th Grade

20 questions
Periodic Trends
Quiz
•
10th Grade

15 questions
Exploring the Unique Properties of Water
Interactive video
•
9th - 12th Grade

17 questions
CHemistry Unit 7 Dimensional Analysis Practice
Quiz
•
9th - 12th Grade

47 questions
Unit #4 Electron KAP Test Review
Quiz
•
10th - 12th Grade

7 questions
Elements, Compounds, Mixtures
Lesson
•
9th - 12th Grade