Chemical Reactions and Ionic Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What type of compound is formed when a metal combines with nonmetals?
Organic compound
Ionic compound
Metallic compound
Covalent compound
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the charge of a potassium ion (K) in the periodic table?
0
+2
+1
-1
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How do you determine the charge of the sulfate ion (SO4)?
By looking at the periodic table
By observing its color
By using a table of polyatomic ions
By measuring its pH
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
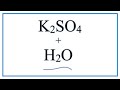
What happens to K2SO4 when it is placed in water?
It evaporates
It forms a precipitate
It remains unchanged
It dissolves into ions
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is a coefficient of 2 placed in front of K+ in the balanced equation?
To balance the charge
To balance the number of potassium ions
To indicate a solid state
To show a reaction with water
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does 'aq' signify when written after an ion in a chemical equation?
The ion is in a liquid state
The ion is dissolved in water
The ion is in a solid state
The ion is in a gaseous state
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is liquid water (H2O) not written on the product side of the equation?
Because it forms a new compound
Because 'aq' already indicates the presence of water
Because it evaporates
Because it is not involved in the reaction
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final equation for the reaction of K2SO4 with water?
K2SO4 + H2O → K2SO4(aq)
K2SO4 + H2O → K2SO4 + H2O
K2SO4 + H2O → 2K+ + SO4^2-
K2SO4 + H2O → K2O + H2SO4
Similar Resources on Wayground

9 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

7 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Copper(II) Bromide and Aqueous Solutions
Interactive video
•
9th - 10th Grade

8 questions
Chemical Reactions and Ionic Compounds
Interactive video
•
9th - 10th Grade

8 questions
Ionic Compounds and Chemical Reactions
Interactive video
•
9th - 10th Grade

11 questions
Net Ionic Equations and Electrolytes
Interactive video
•
9th - 10th Grade

6 questions
Strontium Nitrate and Ion Properties
Interactive video
•
9th - 10th Grade

7 questions
Polyatomic Ions and Ammonium Nitrate
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade