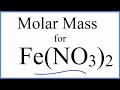
Calculating Molar Mass of Compounds
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Lucas Foster
FREE Resource
Read more
5 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the atomic mass of iron as used in the calculation of Iron II Nitrate's molar mass?
48.00 grams per mole
55.85 grams per mole
14.01 grams per mole
16.00 grams per mole
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are considered in the calculation of Iron II Nitrate's molar mass?
2
3
1
4
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the result of multiplying the atomic mass of oxygen by 3 in the calculation?
32.00 grams per mole
16.00 grams per mole
64.00 grams per mole
48.00 grams per mole
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final molar mass of Iron II Nitrate as calculated in the video?
155.85 grams per mole
179.87 grams per mole
100.00 grams per mole
200.00 grams per mole
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which element's atomic mass is added last in the calculation process?
Hydrogen
Oxygen
Nitrogen
Iron
Access all questions and much more by creating a free account
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

11 questions
Identifying Limiting Reagents in Stoichiometry
Interactive video
•
9th - 12th Grade

11 questions
Molarity and Dilution Equations Challenge
Interactive video
•
9th - 12th Grade

9 questions
Resonance Structures of NO3-
Interactive video
•
9th - 10th Grade

10 questions
Electron Configuration and Atomic Structure
Interactive video
•
9th - 10th Grade

10 questions
Oxidation Numbers in K2Cr2O7
Interactive video
•
9th - 10th Grade

11 questions
Understanding Functions and Inverses
Interactive video
•
9th - 10th Grade

11 questions
Understanding Surface Tension
Interactive video
•
9th - 12th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade