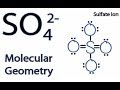
Molecular Geometry and Lewis Structures
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Medium
Amelia Wright
Used 1+ times
FREE Resource
Read more
8 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the central atom in the Lewis structure of SO4 2-?
Oxygen
Nitrogen
Sulfur
Hydrogen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are bonded to the central sulfur atom in SO4 2-?
Five
Four
Two
Three
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the AXN notation for SO4 2-, what does 'X' represent?
Total number of atoms
Non-bonding electron pairs
Number of bonded atoms
Central atom
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the AXN notation for the molecular geometry of SO4 2-?
AX4
AX5
AX3
AX2
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of SO4 2- according to the AX4 notation?
Bent
Tetrahedral
Linear
Trigonal planar
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in a tetrahedral molecular geometry?
180°
120°
109.5°
90°
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Why is it important to consider the three-dimensional structure of SO4 2-?
To accurately describe its shape
To find its mass
To understand its reactivity
To determine the color
8.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What tool can help visualize the three-dimensional shape of SO4 2-?
Periodic table
Lewis structure
Molecular model
Chemical equation
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

6 questions
FOREST Self-Discipline
Lesson
•
1st - 5th Grade

7 questions
Veteran's Day
Interactive video
•
3rd Grade

20 questions
Weekly Prefix check #2
Quiz
•
4th - 7th Grade
Discover more resources for Chemistry

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
2.6 Electron Configurations and Orbital Notations
Quiz
•
10th Grade

17 questions
Protein Synthesis (Protein Synthesis)
Interactive video
•
9th Grade

20 questions
Naming Ionic compounds
Quiz
•
10th Grade

16 questions
Naming Ionic Compounds
Quiz
•
9th - 11th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade

16 questions
Electron Configurations, and Orbital Notations
Quiz
•
9th - 11th Grade

61 questions
Honors Periodic Table Unit Review
Quiz
•
9th - 11th Grade