Molecular Geometry of XO3
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
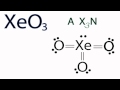
What is the central atom in the XO3 molecule?
Nitrogen
Xenon
Oxygen
Hydrogen
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to VSEPR theory, what causes atoms and lone pairs to spread out in a molecule?
Attraction
Magnetism
Gravity
Repulsion
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the XO3 molecule, where is the lone pair of electrons located?
At the bottom
On the side
On the top
In the center
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What does the 'X' represent in the AXN notation for molecular geometry?
Number of atoms attached
Central atom
Number of lone pairs
Bond angle
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many oxygen atoms are attached to the central Xenon atom in XO3?
Three
One
Four
Two
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the AXN notation for the XO3 molecule?
AX4
AX3N
AX3
AX2
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of XO3 according to the AX3N notation?
Trigonal pyramidal
Trigonal planar
Linear
Tetrahedral
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Recovery Rebate Credit Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding Radical and Quadratic Equations
Interactive video
•
9th - 10th Grade

11 questions
Understanding Trigonometry in Right Triangles
Interactive video
•
9th - 10th Grade

11 questions
Understanding Boiling Points and Intermolecular Forces
Interactive video
•
9th - 10th Grade

11 questions
Chemistry Quiz on Acids and Bases
Interactive video
•
9th - 10th Grade

11 questions
Scientific Notation and Significant Figures Quiz
Interactive video
•
9th - 10th Grade

11 questions
Understanding Atomic Bonds
Interactive video
•
9th - 10th Grade

6 questions
Hubble Space Telescope and Star Formation
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

10 questions
Honoring the Significance of Veterans Day
Interactive video
•
6th - 10th Grade

9 questions
FOREST Community of Caring
Lesson
•
1st - 5th Grade

10 questions
Exploring Veterans Day: Facts and Celebrations for Kids
Interactive video
•
6th - 10th Grade

19 questions
Veterans Day
Quiz
•
5th Grade

14 questions
General Technology Use Quiz
Quiz
•
8th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

15 questions
Circuits, Light Energy, and Forces
Quiz
•
5th Grade

19 questions
Thanksgiving Trivia
Quiz
•
6th Grade
Discover more resources for Chemistry

25 questions
Unit 4/5-Covalent Bonding/Nomenclature
Quiz
•
10th Grade

20 questions
Naming Ionic Compounds
Quiz
•
10th - 12th Grade

20 questions
Ions
Quiz
•
10th Grade

25 questions
VSPER Shape Quiz
Quiz
•
10th Grade

17 questions
Periodic Trends
Quiz
•
10th Grade

61 questions
KAP Chemistry Covalent Test Review
Quiz
•
10th Grade

27 questions
Unit 4/5 Covalent Bonding/Nomenclature
Quiz
•
10th - 12th Grade

21 questions
Naming Covalent and Ionic Compounds
Lesson
•
9th - 12th Grade