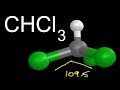
Molecular Geometry of CHCl3
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of using Lewis structures in determining molecular geometry?
To predict the shape of the molecule
To determine the color of the compound
To calculate the molecular weight
To find the melting point
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the AXN notation for CHCl3, what does 'X' represent?
The number of atoms bonded to the central atom
The number of non-bonding electron pairs
The central atom
The total number of atoms in the molecule
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many atoms are bonded to the central carbon in CHCl3?
Five
Four
Three
Two
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of CHCl3 according to the AX4 notation?
Bent
Tetrahedral
Trigonal planar
Linear
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle in a tetrahedral molecular geometry?
109.5°
90°
180°
120°
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the 3D visualization of CHCl3, which atom is represented by the color green?
Oxygen
Carbon
Chlorine
Hydrogen
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Where is the hydrogen atom located in the 3D structure of CHCl3?
At the center
On the side
On the top
On the bottom
Create a free account and access millions of resources
Similar Resources on Wayground

9 questions
Molecular Geometry and Bonding Concepts
Interactive video
•
9th - 10th Grade

9 questions
Geometry and Bonding in CO2
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry and AXN Notation
Interactive video
•
9th - 10th Grade

8 questions
Molecular Geometry of CO2
Interactive video
•
9th - 10th Grade

9 questions
Molecular Geometry of N2O
Interactive video
•
9th - 10th Grade

6 questions
Molecular Geometry of AsF5
Interactive video
•
9th - 10th Grade

11 questions
Molecular Geometry of H2SO4
Interactive video
•
9th - 10th Grade

10 questions
Acetylene Molecular Geometry and Bonding
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

12 questions
Unit Zero lesson 2 cafeteria
Lesson
•
9th - 12th Grade

10 questions
Nouns, nouns, nouns
Quiz
•
3rd Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

11 questions
All about me
Quiz
•
Professional Development

20 questions
Lab Safety and Equipment
Quiz
•
8th Grade

13 questions
25-26 Behavior Expectations Matrix
Quiz
•
9th - 12th Grade

10 questions
Exploring Digital Citizenship Essentials
Interactive video
•
6th - 10th Grade
Discover more resources for Chemistry

20 questions
Lab Safety and Lab Equipment
Quiz
•
9th - 12th Grade

20 questions
Lab Equipment Quiz Chemistry
Quiz
•
9th - 12th Grade

12 questions
elements, compounds, and mixtures
Quiz
•
9th Grade

19 questions
Lab Safety & Lab Equipment
Quiz
•
10th Grade

30 questions
ACA Unit 1 Atomic Structure
Quiz
•
9th - 12th Grade

21 questions
Lab Safety
Quiz
•
10th Grade

20 questions
States of Matter and Phase Changes
Quiz
•
9th - 12th Grade

8 questions
Metric System
Lesson
•
9th - 12th Grade