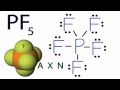
Molecular Geometry of PF5
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Practice Problem
•
Hard
Amelia Wright
FREE Resource
Read more
10 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the first step in determining the molecular geometry of PF5?
Measuring bond angles
Using the AXN notation
Counting non-bonding electron pairs
Looking at the Lewis structure
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
According to VSEPR theory, how do fluorine atoms arrange themselves around the phosphorus atom in PF5?
In a square planar arrangement
In a linear arrangement
As close to each other as possible
As far apart as possible while remaining bonded
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the molecular geometry of PF5 as determined by the AXN notation?
Trigonal bipyramidal
Tetrahedral
Trigonal planar
Octahedral
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
In the AXN notation for PF5, what does 'X' represent?
The total number of electron pairs
The number of atoms bonded to the central atom
The number of non-bonding electron pairs
The central atom
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle between the equatorial fluorine atoms in PF5?
180°
120°
90°
109.5°
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the bond angle between the axial and equatorial fluorine atoms in PF5?
109.5°
180°
120°
90°
7.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which of the following is NOT a feature of the trigonal bipyramidal geometry?
Five bonded atoms
Two different bond angles
A central atom with non-bonding pairs
Equatorial and axial positions
Create a free account and access millions of resources
Create resources
Host any resource
Get auto-graded reports

Continue with Google

Continue with Email

Continue with Classlink

Continue with Clever
or continue with

Microsoft
%20(1).png)
Apple
Others
Already have an account?
Similar Resources on Wayground

6 questions
Mayor Jimmy Walker presents Medal of the City of New York to Dino Grandi
Interactive video
•
9th - 10th Grade

5 questions
GCSE Secondary Maths Age 13-17 - Geometry & Measures: Volume - Explained
Interactive video
•
9th - 10th Grade

3 questions
Learn how to find the missing angle measure given arc length and radius
Interactive video
•
9th - 10th Grade

6 questions
CLEAN : Producers of James Bond place handprints on Hollywood Boulevard
Interactive video
•
9th - 12th Grade

8 questions
How to Find Angles Formed by Parallel Lines and a Transversal
Interactive video
•
10th - 12th Grade

6 questions
GCSE Secondary Maths Age 13-17 - Geometry & Measures: Area - Explained
Interactive video
•
10th - 12th Grade

6 questions
GCSE Secondary Maths Age 13-17 - Geometry & Measures: Construction - Explained
Interactive video
•
10th - 12th Grade

8 questions
Dianne Bilyak "A Bell of Water, Ringing"
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

5 questions
This is not a...winter edition (Drawing game)
Quiz
•
1st - 5th Grade

25 questions
Multiplication Facts
Quiz
•
5th Grade

10 questions
Identify Iconic Christmas Movie Scenes
Interactive video
•
6th - 10th Grade

20 questions
Christmas Trivia
Quiz
•
6th - 8th Grade

18 questions
Kids Christmas Trivia
Quiz
•
KG - 5th Grade

11 questions
How well do you know your Christmas Characters?
Lesson
•
3rd Grade

14 questions
Christmas Trivia
Quiz
•
5th Grade

20 questions
How the Grinch Stole Christmas
Quiz
•
5th Grade