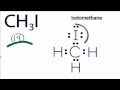
Lewis Structure of CH3I
Interactive Video
•
Chemistry
•
9th - 10th Grade
•
Hard
Aiden Montgomery
FREE Resource
Read more
6 questions
Show all answers
1.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the total number of valence electrons in CH3I?
18
12
14
16
2.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
Which atom is placed at the center of the CH3I Lewis structure?
Carbon
Iodine
Oxygen
Hydrogen
3.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons are used to form chemical bonds in CH3I?
10
8
6
12
4.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the purpose of adding electrons around iodine in the CH3I structure?
To increase its electronegativity
To fill its octet
To change its oxidation state
To make it more reactive
5.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
How many valence electrons does carbon have around it in the completed CH3I structure?
8
10
6
4
6.
MULTIPLE CHOICE QUESTION
30 sec • 1 pt
What is the final step in completing the Lewis structure of CH3I?
Changing the central atom
Removing excess electrons
Ensuring all atoms have complete octets
Adding more hydrogen atoms
Similar Resources on Wayground

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
ICl3 Molecular Structure and Bonding
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons and Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Valence Electrons in AsCl3
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structure of SeBr2
Interactive video
•
9th - 10th Grade

6 questions
SCl2 Molecular Structure and Electrons
Interactive video
•
9th - 10th Grade

6 questions
Bromine and Chlorine Lewis Structures
Interactive video
•
9th - 10th Grade

6 questions
Lewis Structures and Valence Electrons
Interactive video
•
9th - 10th Grade
Popular Resources on Wayground

18 questions
Writing Launch Day 1
Lesson
•
3rd Grade

11 questions
Hallway & Bathroom Expectations
Quiz
•
6th - 8th Grade

11 questions
Standard Response Protocol
Quiz
•
6th - 8th Grade

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

4 questions
Exit Ticket 7/29
Quiz
•
8th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade
Discover more resources for Chemistry

40 questions
Algebra Review Topics
Quiz
•
9th - 12th Grade

10 questions
Lab Safety Procedures and Guidelines
Interactive video
•
6th - 10th Grade

19 questions
Handbook Overview
Lesson
•
9th - 12th Grade

20 questions
Subject-Verb Agreement
Quiz
•
9th Grade

24 questions
Scientific method and variables review
Quiz
•
9th Grade

10 questions
Characteristics of Life
Quiz
•
9th - 10th Grade

19 questions
Mental Health Vocabulary Pre-test
Quiz
•
9th Grade

14 questions
Points, Lines, Planes
Quiz
•
9th Grade